Rhodium-Catalyzed One-Carbon Ring Expansion of Aziridines with Vinyl-N-triftosylhydrazones for the Synthesis of 2-Vinyl Azetidines
Graphical Abstract
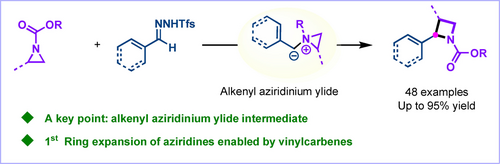
A general skeletal ring expansion strategy for the direct conversion of aziridines into 2-vinyl azetidines via one-carbon insertion using vinyl-N-triftosylhydrazones is described. The method is scalable, tolerates diverse functional groups, and is amenable to the synthesis of medicinally relevant molecules.
Abstract
Azetidines, being four-membered N-heterocycles, possess significant potential in contemporary medicinal chemistry owing to their favorable pharmacokinetic properties. Regrettably, the incorporation of functionalized azetidines into pharmaceutical lead structures has been impeded by the absence of efficient synthetic methods for their synthesis. In this study, a Rh-catalyzed one-carbon ring expansion of aziridines with vinyl-N-triftosylhydrazones is presented, which facilitates the synthesis of high value-added 2-alkenyl azetidine products. This research represents the first example of ring expansion of aziridines enabled by vinyl carbenes. Additionally, a one-pot two-step protocol, initiated from cinnamaldehyde, was successfully achieved, offering a step-economical and facile approach for the synthesis of these compounds. The pivotal aspect of this successful transformation lies in the in situ formation of an alkenyl aziridinium ylide intermediate. Experimental investigations, coupled with computational studies, suggest that a diradical pathway is involved in the reaction mechanism.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.