Cover Picture: Tailoring Electrochemical CO2 Reduction on Copper by Reactive Ionic Liquid and Native Hydrogen Bond Donors (Angew. Chem. Int. Ed. 1/2024)
Graphical Abstract
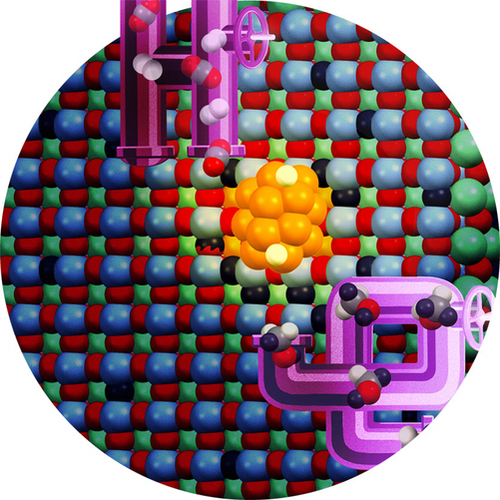
The dynamic interplay between a copper electrode and a reactive ionic liquid is illustrated in the cover picture, showcasing key components in the electrochemical reduction of CO2. The role of the cation in covering the electrode surface and the in-situ generated hydrogen bond donor from CO2 binding to the ionic liquid in enhancing the kinetics were uncovered by in-situ spectroscopy, complemented by electroanalytical and computational methods. Multi-carbon products with reduced reaction energy were obtained, as reported by Burcu Gurkan et al. in their Research Article (e202312163). Cover image credit: Miguel Munoz.
The dynamic interplay between a copper electrode and a reactive ionic liquid is illustrated in the cover picture, showcasing key components in the electrochemical reduction of CO2. The role of the cation in covering the electrode surface and the in-situ generated hydrogen bond donor from CO2 binding to the ionic liquid in enhancing the kinetics were uncovered by in-situ spectroscopy, complemented by electroanalytical and computational methods. Multi-carbon products with reduced reaction energy were obtained, as reported by Burcu Gurkan et al. in their Research Article (e202312163). Cover image credit: Miguel Munoz.
Depolymerization
Self-Assembly
Heterogeneous Catalysis