Domino-Redox Reaction Induced by An Electrochemically Triggered Conformational Change
Graphical Abstract
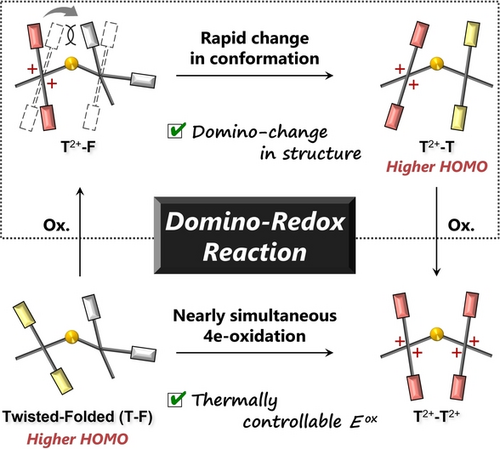
A thermally activated twisted-folded (T-F) form of bisquinodimethane with a dithiin skeleton (SS-BQD) undergoes a facile apparent 2e-transfer (trigger). Furthermore, in the resulting dication, the steric repulsion and interelectrophore interaction cause a facile and rapid change in the structure from the as-generated T2+-F form to the T2+-T form (domino), which facilitates the subsequent oxidation to the tetracation (domino).
Abstract
The concept of a domino-type reaction has been applied in a wide range of fields such as synthetic organic chemistry, material engineering, and life science. To extend the domino concept to redox chemistry, we designed and synthesized a dimeric quinodimethane (QD) with a nonplanar dithiin spacer. The domino-redox properties can be activated by raising the temperature, based on a thermally equilibrated twisted conformation of QD, which has a higher HOMO level that is more readily oxidized. After one QD unit is oxidized (trigger), steric repulsion and electronic interaction between electrophores make the neighboring QD unit adopt a twisted conformation (domino process), which facilitates the following oxidation. Thus, a domino-redox reaction was achieved for the first time by a change in the HOMO level due to a drastic change in the molecular conformation.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.