Enantioselective Palladium(II)-Catalyzed Desymmetrizative Coupling of 7-Azabenzonorbornadienes with Alkynylanilines
Junjie Meng
National Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641 Guangdong, China
These authors contributed equally to this work
] co-first authors
Search for more papers by this authorHui He
Department of Chemistry, Shantou University, Shantou, 515063 Guangdong, China
These authors contributed equally to this work
] co-first authors
Search for more papers by this authorQianru Liu
National Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641 Guangdong, China
Search for more papers by this authorProf. Hanhong Xu
National Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641 Guangdong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Huicai Huang
Key Laboratory of Chinese Medicinal Resource from Lingnan, Ministry of Education, School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, 510641 Guangdong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shao-Fei Ni
Department of Chemistry, Shantou University, Shantou, 515063 Guangdong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhaodong Li
National Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641 Guangdong, China
National Key Laboratory on Technologies for Chinese Medicine Pharmaceutical Process Control and Intelligent Manufacture, School of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, 210023 Jiangsu, China
Search for more papers by this authorJunjie Meng
National Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641 Guangdong, China
These authors contributed equally to this work
] co-first authors
Search for more papers by this authorHui He
Department of Chemistry, Shantou University, Shantou, 515063 Guangdong, China
These authors contributed equally to this work
] co-first authors
Search for more papers by this authorQianru Liu
National Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641 Guangdong, China
Search for more papers by this authorProf. Hanhong Xu
National Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641 Guangdong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Huicai Huang
Key Laboratory of Chinese Medicinal Resource from Lingnan, Ministry of Education, School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, 510641 Guangdong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shao-Fei Ni
Department of Chemistry, Shantou University, Shantou, 515063 Guangdong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhaodong Li
National Key Laboratory of Green Pesticide, College of Materials and Energy, South China Agricultural University, Guangzhou, 510641 Guangdong, China
National Key Laboratory on Technologies for Chinese Medicine Pharmaceutical Process Control and Intelligent Manufacture, School of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, 210023 Jiangsu, China
Search for more papers by this authorThese authors contributed equally to this work
] co-first authors
Graphical Abstract
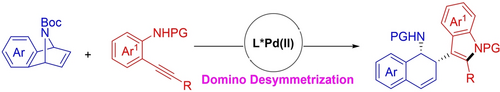
A palladium-catalyzed enantioselective desymmetrizative coupling of prochiral 7-azabenzonorbornadienes with readily available alkynylanilines afforded dihydronaphthalenes via concomitant generation of three covalent bonds. The methods also allows to build two stereocenters and an indole motif in a highly diastereo- and enantioselective as well as atom-economic manner.
Abstract
A PdII-catalyzed, domino enantioselective desymmetrizative coupling of 7-azabenzonorbornadienes with alkynylanilines is disclosed herein. This operationally simple transformation generates three covalent bonds and two contiguous stereocenters with excellent enantio- and diastereo-selectivity. The resulting functionalized indole-dihydronaphthalene-amine conjugates served as an appealing platform to streamline the diversity-oriented synthesis (DOS) of other valuable enantioenriched compounds. DFT calculations revealed that the two stabilizing non-covalent interactions contributed to the observed enantioselectivity.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202315092-sup-0001-misc_information.pdf36 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aX.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou, J. Zhou, Chem. Rev. 2016, 116, 7330–7396;
- 1bC. Nájera, F. Foubelo, J. M. Sansano, M. Yus, Tetrahedron 2022, 106, 132629;
- 1cJ. Sietmann, J. M. Wahl, Angew. Chem. Int. Ed. 2020, 59, 6964–6974.
- 2
- 2aM. Pineschi, Eur. J. Org. Chem. 2006, 4979–4988;
- 2bD. Zhang, R. Khan, B. Fan, ChemistrySelect 2020, 5, 5526–5536;
- 2cM. J. Fleming, M. Lautens, in Catalyzed Carbon-Heteroatom Bond Formation (Eds.: A. K. Yudin), Wiley-VCH, Weinheim, 2010, pp 411–436;
10.1002/9783527633388.ch10 Google Scholar
- 2dM. Pineschi, Eur. J. Org. Chem. 2020, 2643–2649.
- 3For selected examples, see:
- 3aF. Menard, M. Lautens, Angew. Chem. Int. Ed. 2008, 47, 2085–2088;
- 3bJ. John, I. U, E. Suresh, K. V. Radhakrishnan, J. Am. Chem. Soc. 2009, 131, 5042–5043;
- 3cS. Crotti, F. Bertolini, F. Macchiaa, M. Pineschi, Chem. Commun. 2008, 3127–3129;
- 3dH. Lee, J. T. Han, J. Yun, ACS Catal. 2016, 6, 6487–6490;
- 3eY. Zhang, Q. Wu, S. Cui, Chem. Sci. 2014, 5, 297–302;
- 3fS.-G. Wang, N. Cramer, Angew. Chem. Int. Ed. 2019, 58, 2514–2518.
- 4
- 4aJ. P. Freeman, E. T. Michalson, S. V. D'Andrea, L. Baczynskyj, P. F. Von Voigtlander, R. A. Lahti, M. W. Smith, C. F. Lawson, T. A. Scahill, S. A. Mizsak, J. Szmuszkovicz, J. Med. Chem. 1991, 34, 1891–1896;
- 4bP. Rajagopalan, R. M. Scribner, P. Pennev, W. K. Schmidt, S. W. Tam, G. F. Steinfels, L. Cook, Bioorg. Med. Chem. Lett. 1992, 2, 715–720;
- 4cM. J. Fleming, H. A. McManus, A. Rudolph, W. H. Chan, J. Ruiz, C. Dockendorff, M. Lautens, Chem. Eur. J. 2008, 14, 2112–2124.
- 5For selected reviews, see:
- 5aM. Lautens, K. Fagnou, S. Hiebert, Acc. Chem. Res. 2003, 36, 48–58;
- 5bD. K. Rayabarapu, C.-H. Cheng, Acc. Chem. Res. 2007, 40, 971–983;
- 5cG. Hilt, Angew. Chem. Int. Ed. 2009, 48, 6390–6393;
- 5dF. Wang, S. Yu, X. Li, Chem. Soc. Rev. 2016, 45, 6462–6477;
- 5eS. V. Kumar, A. Yen, M. Lautens, P. J. Guiry, Chem. Soc. Rev. 2021, 50, 3013–3093.
- 6For selected examples, see:
- 6aD. K. Rayabarapu, C.-F. Chiou, C.-H. Cheng, Org. Lett. 2002, 4, 1679–1682;
- 6bD. K. Rayabarapu, C.-H. Cheng, Chem. Eur. J. 2003, 9, 3164–3169;
- 6cL. Zhang, C. M. Le, M. Lautens, Angew. Chem. Int. Ed. 2014, 53, 5951–5954;
- 6dS. Mannathana, C.-H. Cheng, Adv. Synth. Catal. 2014, 356, 2239–2246;
- 6eJ. Chen, L. Zou, C. Zeng, Y. Zhou, B. Fan, Org. Lett. 2018, 20, 1283–1286;
- 6fX. Zhang, Y. Gao, J. Chen, R. Fan, G. Shi, Z. He, B. Fan, Adv. Synth. Catal. 2019, 361, 4495–4499;
- 6gY. Luo, Á. Gutierrez-Bonet, J. K. Matsui, M. E. Rotella, R. Dykstra, O. Gutierrez, G. A. Molander, ACS Catal. 2019, 9, 8835–8842;
- 6hA. G. Diallo, D. Roy, S. Gaillard, M. Lautens, J.-L. Renaud, Org. Lett. 2020, 22, 2442–2447;
- 6iK. Zhang, R Khan, J. Chen, X. Zhang, Y. Gao, Y. Zhou, K. Li, Y. Tian, B. Fan, Org. Lett. 2020, 22, 3339–3344;
- 6jD. Ding, B. Yuan, H. Wen, C. Wang, Cell Rep. Phys. Sci. 2023, 4, 101166.
- 7
- 7aA. J. Kochanowska-Karamyan, M. T. Hamann, Chem. Rev. 2010, 110, 4489–4497;
- 7bH.-H. Zhang, F. Shi, Acc. Chem. Res. 2022, 55, 2562–2580.
- 8
- 8aZ. Qi, X. Li, Angew. Chem. Int. Ed. 2013, 52, 8995–9000;
- 8bX. Yang, G. Zheng, X. Li, Angew. Chem. Int. Ed. 2019, 58, 322–326.
- 9
- 9aS. Li, Z. Wang, H. Xiao, Z. Bian, J. Wang, Chem. Commun. 2020, 56, 7573–7576;
- 9bY. Zheng, W.-Y. Zhang, Q. Gu, C. Zheng, S.-L. You, Nat. Commun. 2023, 14, 1094.
- 10For selected reviews on domino reactions, see:
- 10aL. F. Tietze, Chem. Rev. 1996, 96, 115–136;
- 10bH. Pellissier, Curr. Org. Chem. 2021, 25, 1457–1471;
- 10cA. Pounder, E. Neufeld, P. Myler, W. Tam, Beilstein J. Org. Chem. 2023, 19, 487–540.
- 11For selected reviews, see:
- 11aS. Cacchi, G. Fabrizi, Chem. Rev. 2005, 105, 2873–2920;
- 11bG. Zeni, R. C. Larock, Chem. Rev. 2006, 106, 4644–4680;
- 11cS. Cacchi, G. Fabrizi, Chem. Rev. 2011, 111, PR215 - PR283;
- 11dR. I. McDonald, G. Liu, S. S. Stahl, Chem. Rev. 2011, 111, 2981–3019;
- 11eX. Han, X. Lu, Synlett 2018, 29, 2461–2480;
- 11fJ. Li, S. Yang, W. Wu, H. Jiang, Chem. Asian J. 2019, 14, 4114–4128;
- 11gY. Ping, Y. Li, J. Zhu, W. Kong, Angew. Chem. Int. Ed. 2019, 58, 1562–1573;
- 11hM. Liu, J. Sun, K. M. Engle, Tetrahedron 2022, 103, 132513.
- 12For selected examples, see:
- 12aX. Han, X. Lu, Org. Lett. 2010, 12, 3336–3339;
- 12bC. Feng, T.-P. Loh, J. Am. Chem. Soc. 2010, 132, 17710–17712;
- 12cB. Yao, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2012, 51, 12311–12315;
- 12dB. Yao, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2012, 51, 5170–5174;
- 12eB. Yao, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2013, 52, 12992–12996;
- 12fZ. Hu, J. Wang, D. Liang, Q. Zhu, Adv. Synth. Catal. 2013, 355, 3290–3294;
- 12gQ. Wang, L. Huang, X. Wu, H. Jiang, Org. Lett. 2013, 15, 5940–5943;
- 12hB. Yao, Q. Wang, J. Zhu, Chem. Eur. J. 2014, 20, 12255–12261;
- 12iG. Xia, X. Han, X. Lu, Org. Lett. 2014, 16, 2058–2061;
- 12jG. Xia, X. Han, X. Lu, Org. Lett. 2014, 16, 6184–6187;
- 12kK. Yuan, L. Liu, J. Chen, S. Guo, H. Yao, A. Lin, Org. Lett. 2018, 20, 3477–3481;
- 12lJ. Chen, X. Han, X. Lu, Org. Lett. 2018, 20, 7470–7473;
- 12mR. K. Shukla, A. K. Chaturvedi, C. M. R. Volla, ACS Catal. 2021, 11, 7750–7761;
- 12nW.-C. Yang, X.-B. Chen, K.-L. Song, B. Wu, W.-E. Gan, Z.-J. Zheng, J. Cao, L.-W. Xu, Org. Lett. 2021, 23, 1309–1314;
- 12oY. Wu, B. Xu, G. Zhao, Z. Pan, Z.-M. Zhang, J. Zhang, Chin. J. Chem. 2021, 39, 3255–3260;
- 12pX. Yang, G. Wang, Z.-S. Ye, Chem. Sci. 2022, 13, 10095–10102;
- 12qW. Yuan, X. Li, Z. Qi, X. Li, Org. Lett. 2022, 24, 2093–2098;
- 12rR. Arora, J. F. Rodríguez, A. Whyte, M. Lautens, Angew. Chem. Int. Ed. 2022, 61, e202112288.
- 13J. Chen, X. Han, X. Lu, Angew. Chem. Int. Ed. 2017, 56, 14698–14701.
- 14X.-D. Hu, Z.-H. Chen, J. Zhao, R.-Z. Sun, H. Zhang, X. Qi, W.-B. Liu, J. Am. Chem. Soc. 2021, 143, 3734–3740.
- 15For application in axially chiral synthesis, see:
- 15aK. Kamikawa, S. Kinoshita, M. Furusyo, S. Takemoto, H. Matsuzaka, M. Uemura, J. Org. Chem. 2007, 72, 3394–3402;
- 15bN. Ototake, Y. Morimoto, A. Mokuya, H. Fukaya, Y. Shida, O. Kitagawa, Chem. Eur. J. 2010, 16, 6752–6755;
- 15cM. Tian, D. Bai, G. Zheng, J. Chang, X. Li, J. Am. Chem. Soc. 2019, 141, 9527–9532;
- 15dY.-P. He, H. Wu, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2020, 59, 2105–2109;
- 15eX. Li, L. Zhao, Z. Qi, X. Li, Org. Lett. 2021, 23, 5901–5905;
- 15fC.-S. Wang, L. Wei, C. Fu, X.-H. Wang, C.-J. Wang, Org. Lett. 2021, 23, 7401–7406.
- 16
- 16aH. Wu, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2018, 57, 2721–2725;
- 16bY.-P. He, J. Cao, H. Wu, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2021, 60, 7093–7097;
- 16cJ. Cao, H. Wu, Q. Wang, J. Zhu, Nat. Chem. 2021, 13, 671–676.
- 17
- 17aZ. Shao, S. Zhang, Y. Chen, Y.-L. Liu, R.-Y. Tang, Z. Li, Tetrahedron 2020, 76, 131199;
- 17bY. Zhang, Z. Ren, Y.-L. Liu, Z. Wang, Z. Li, Eur. J. Org. Chem. 2020, 5192–5200;
- 17cC. Fu, Z. Zhang, Y. Li, D. Gao, Z.-N. Cui, Z. Li, Chem. Commun. 2022, 58, 5614–5617;
- 17dY. Du, S. Chen, A. Huang, Y. Chen, Y.-L. Liu, G. Song, R.-Y. Tang, H. Xu, G. Yao, Z. Li, Org. Lett. 2022, 24, 1341–1345;
- 17eY. Du, S. Chen, H. Cao, Y. Zhang, H. Lei, G. Xia, H. Huang, Z. Li, Org. Lett. 2023, 25, 2218–2222.
- 18
- 18aJ. E. Perea-Buceta, T. Wirtanen, O.-V. Laukkanen, M. K. Mäkelä, M. Nieger, M. Melchionna, N. Huittinen, J. A. Lopez-Sanchez, J. Helaja, Angew. Chem. Int. Ed. 2013, 52, 11835–11839;
- 18bJ. Sun, K. Wang, P. Wang, G. Zheng, X. Li, Org. Lett. 2019, 21, 4662–4666.
- 19For a review on chiral ferrocene ligands, see: M. Drusan, R. Šebesta, Tetrahedron 2014, 70, 759–786.
- 20Deposition numbers 2292475 (for 9), 2292476 (for 38), and 2292474 (for 39) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 21
- 21aJ. Lv, B. Zhao, L. Liu, Y. Han, Y. Yuan, Z. Shi, Adv. Synth. Catal. 2018, 360, 4054–4059;
- 21bC.-H. Yang, Y.-S. Zhang, W.-W. Fan, G.-Q. Liu, Y.-M. Li, Angew. Chem. Int. Ed. 2015, 54, 12636–12639.
- 22
- 22aT.-S. Mei, E. W. Werner, A. J. Burkle, M. S. Sigman, J. Am. Chem. Soc. 2013, 135, 6830–6833;
- 22bC. Zhang, C. B. Santiago, J. M. Crawford, M. S. Sigman, J. Am. Chem. Soc. 2015, 137, 15668–15671.
- 23T. J. Seguin, T. Lu, S. E. Wheeler, Org. Lett. 2015, 17, 3066–3069.
- 24
- 24aP. Maity, R. P. Pemberton, D. J. Tantillo, U. K. Tambar, J. Am. Chem. Soc. 2013, 135, 16380–16383;
- 24bJ.-Y. Zhang, J.-Y. Chen, C.-H. Gao, L. Yu, S.-F. Ni, W. Tan, F. Shi, Angew. Chem. Int. Ed. 2023, 62, e202305450.
- 25Y. Tian, Y.-Y. Zhu, J. Yu, D.-H. Liu, Q. Yin, S.-F. Ni, S.-T. Bai, X. Zhang, CCS Chem. 2023 https://doi.org/10.31635/ccschem.023.202202601.