Increasing Chemical Diversity of B2N2 Anthracene Derivatives by Introducing Continuous Multiple Boron-Nitrogen Units
Graphical Abstract
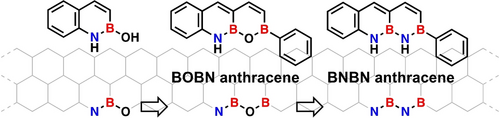
A synthetic approach has been developed to introduce multiple main group heteroatoms at the zigzag edge of the anthracene framework. BN annulation has led to a naphthalene derivative with an NBO bond. A subsequent BO annulation has been used to extend the fused aromatic ring to afford an anthracene derivative featuring an NBOB system. Changing the NBOB to an NBNB system has further increased the chemical diversity.
Abstract
Increasing the chemical diversity of organic semiconductors is essential to develop efficient electronic devices. In particular, the replacement of carbon-carbon (C−C) bonds with isoelectronic boron-nitrogen (B−N) bonds allows precise modulation of the electronic properties of semiconductors without significant structural changes. Although some researchers have reported the preparation of B2N2 anthracene derivatives with two B−N bonds, no compounds with continuous multiple BN units have been prepared yet. Herein, we report the synthesis and characterization of a B2N2 anthracene derivative with a BNBN unit formed by converting the BOBN unit at the zigzag edge. Compared to the all-carbon analogue 2-phenylanthracene, BNBN anthracene exhibits significant variations in the C−C bond length and a larger highest occupied molecular orbital–lowest unoccupied molecular orbital energy gap. The experimentally determined bond lengths and electronic properties of BNBN anthracene are confirmed through theoretical calculations. The BOBN anthracene organic light-emitting diode, used as a blue host, exhibits a low driving voltage. The findings of this study may facilitate the development of larger acenes with multiple BN units and potential applications in organic electronics.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.