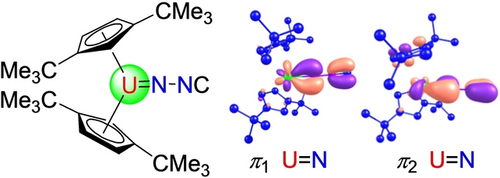
Experimental and Computational Studies on Uranium Diazomethanediide Complexes
Graphical Abstract
In this contribution, the synthesis, structure, and reactivity of metal (uranium) diazomethanediide complexes containing bridging and/or terminal isocyanoimido groups (U=NNC) are presented. By a combined experimental and computational study, unique reactivity patterns were identified and this gained knowledge provides the first insights into the reactivity of complexes containing a metal isocyanoimido (M=NNC) fragment.
Abstract
Uranium diazomethanediide complexes can be prepared and their synthesis, structure and reactivity were explored. Reaction of the uranium imido compound [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (1) or [η5-1,3-(Me3C)2C5H3]2U=N(p-tolyl)(dmap) (4) with Me3SiCHN2 cleanly yields the first isocyanoimido metal complexes [η5-1,2,4-(Me3Si)3C5H2]2U(=NNC)(μ-CNN=)U(dmap)[η5-1,2,4-(Me3Si)3C5H2]2 (2) and {[η5-1,3-(Me3C)2C5H3]2U[μ-(=NNC)]}6 (5), respectively. Both compounds exhibit remarkable thermal stability and were fully characterized. According to density functional theory (DFT) studies the bonding between the Cp2U2+ and [NNC]2− moieties is strongly polarized with a significant 5 f orbital contribution, which is also reflected in the reactivity of these complexes. For example, complex 5 acts as a nucleophile toward alkylsilyl halides and engages in a [2+2] cycloaddition with CS2, but no reaction occurs in the presence of internal alkynes.
Introduction
Diazoalkanes R(R′)CNN are important building blocks acting either as 1,3-dipoles in cycloaddition reactions or as a C1 source transferring R(R′)C: concomitant with N2 elimination.1, 2 From an inorganic perspective diazoalkanes exhibit not only a rich coordination chemistry, but they may also react after N2 release as carbene R(R′)C: transfer reagents to metal atoms forming metal alkylidene complexes.3 Furthermore metal nitrilimides (M-NNCR) may engage in N-atom transfer reactions, releasing cyanide instead of N2.4 More systematic studies, however, on this reactivity are required and only a small number of nitrilimide complexes have so far been structurally authenticated.4b-5 Nevertheless, returning to diazoalkanes, compounds featuring the parent diazomethanide (HCNN−) or the doubly deprotonated diazomethanediide (CNN2−; Scheme 1) have remained elusive until recently.6, 7 This is surprising since the reports on metal(oid) (RnM)2CN2 (M=Si, Ge, Sn, Pb, Hg, Cd, Ag) complexes date back to 1963.6 While vibrational spectroscopy suggested a μ-κC : κC-structural motive, further characterization suffers from their highly explosive nature as exemplified by Ag2(CN2).6e More recent investigations by Munz and Meyer implicated a cobalt(II)-κN-diazomethanediide intermediate complex as transient intermediate in a C−N bond cleavage process.4c Holthausen and Schneider finally prepared platinum(II) compounds with a terminal or a bridging CNN2− ligand.7 In the monomeric species κN-coordination of the CNN2− ligand is realized, whereas κN- and κC-coordination is found in the dimer with a bridging CNN2− ligand (Scheme 1, A and B). However, diazomethanediide complexes featuring a terminal isocyanoimido (M=NNC) (Scheme 1, C) or diazocarbene (M=CN2) ligand (Scheme 1, D) with metal-ligand multiple bonds have not been reported so far. For many years we have now been interested in actinide complexes featuring actinide-element multiple bonds,8, 9 so we wondered if we would be able to contribute to this interesting, but hitherto unknown class of compounds. Gratifyingly during our recent investigations on terminal imido actinide complexes,8, 9 we indeed succeeded to prepare the first isocyanoimido uranium complexes and to probe their reactivity. These results are reported in this article.
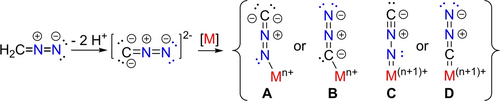
Possible products for the metalation of parent diazomethane.
Results and Discussion
Synthesis of Uranium Isocyanoimido Complexes
Heating a toluene solution of (trimethylsilyl)diazomethane, Me3SiCHN2, and the uranium terminal imido metallocene [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (1) at 80 °C, gives after recrystallization from an n-hexane solution the bimetallic isocyanoimido uranium complex [η5-1,2,4-(Me3Si)3C5H2]2U(=NNC)(μ-CNN=)U(dmap)[η5-1,2,4-(Me3Si)3C5H2]2 (2) in 76 % isolated yield (Scheme 2). Furthermore, complex 2 shows notable thermal stability with a decomposition temperature in the solid state above 230 °C. Two mechanistic scenarios accounting for the formation of 2 can be envisioned: (A) Similar to the reactivity of the thorium imido metallocene [η5-1,2,4-(Me3C)3C5H2]2Th=N(p-tolyl) with Me3SiCHN2,8d an initial deprotonation occurs between 1 and Me3SiCHN2 yielding a nitrilimido intermediate concomitant with dmap release (Scheme 2). In a second step, the nitrilimido complex converts via p-tolylNHSiMe3 elimination to a terminal isocyanoimido complex, which dimerizes to the product 2 in the presence of dmap (Scheme 2; left). (B) Alternatively, after formation of the nitrilimido intermediate a [1,3]-Si migration may occur to yield an unstable isocyanoamido intermediate,5b, 7, 10 eliminating p-tolylNHSiMe3 to give the terminal isocyanoimido compound, which in the presence of dmap readily dimerizes to the final product 2 (Scheme 2; right). To evaluate the feasibility of both pathways, DFT studies were performed. After initial dmap dissociation from 1 the imido intermediate INTa is formed (Figure 1). Coordination of Me3SiCHN2 to INTa forms the adduct COM, followed by intramolecular deprotonation via transition state TSa to yield the nitrilimido intermediate INTb. The two sterically encumbered 1,2,4-(Me3Si)3C5H2 ligands prevent the [1,3]-Si migration, but instead INTb eliminates p-tolylNHSiMe3 via the transition state TSb to give a terminal isocyanoimido intermediate INTc, which gives in the presence of dmap the product 2. The formation of 2 is exergonic with ΔG(353 K)=−120.8 kJ mol−1 and the reaction barrier is ΔG≠(353 K)=125.8 kJ mol−1 (Figure 1), that can be overcome under the experimental reaction conditions. Furthermore, DFT computations also predict that the dimer formation is energetically much more favorable than a hypothetic monomeric dmap adduct [η5-1,2,4-(Me3Si)3C5H2]2U(=NNC)(dmap) (2′) (ΔG(353 K)=−95.7 kJ mol−1) (see the Supporting Information for details), which is also consistent with the experiment. The molecular structure of 2 is shown in Figure 2, and represents, to the best of our knowledge, the first structurally authenticated isocyanoimido complex. The dimeric complex 2 contains a μ-κN : κC bridging isocyanoimido group and a terminal κN-isocyanoimido ligand. The U(1) atom is η5-bound to two eclipsed Cp-rings, and it coordinates κN to the terminal nitrogen atom of a [NNC]2− group and κC to the carbon atom of the other [CNN]2− group in a distorted-tetrahedral fashion. The average U(1)−C(ring) distance is 2.788(15) Å, and the Cp(cent)−U(1)−Cp(cent) and N(1)−U(1)−C(58) angles are 132.8(2)° and 88.6(2)° (Table 1), respectively. The U(2) atom is also η5-coordinate to two eclipsed Cp-rings, but it is bound to two nitrogen atoms, one nitrogen atom of a [NNC]2− group and the pyridine nitrogen atom of the dmap ligand forming a distorted-tetrahedral structure. The average U(2)-C(ring) distance of 2.786(15) Å is essentially identical to that at U(1), whereas slight deviations are observed for the Cp(cent)−U(2)−Cp(cent) and N(3)−U(2)−N(5) angles of 131.1(2)° and 92.6(2)°, respectively. The short U(1)−N(1) and U(2)−N(3) distances of 2.035(4) Å and 2.067(4) Å, respectively, are comparable to the U=N distances found in the uranium imido complex 1 (2.021(5) Å),9 and the diazoalkyl compounds [(2-iPr2P-4-MePh)2N]2U=N2CPh2 (2.097(5) Å),11a (η5-C5Me5)2U(=N2CPh2)2 (1.999(4) and 2.018(4) Å),11a [iPrNC(NiPr2)NiPr]3U=N2CPh2 (2.060(3) Å),11b and [η5-1,2,4-(Me3Si)3C5H2]2U(bipy)(=N2CHSiMe3) (2.014(4) Å).11c Nevertheless, the significantly longer U(2)-N(5) distance of 2.481(5) Å between the dmap ligand and the U(IV) atom is comparable to the U−N(dmap) distance established in 1 (2.426(6) Å),9 which is in line with a dative bond. Furthermore, the U(1)−C(58) distance of 2.548(5) Å is markedly longer than the U−C(Me) bonds found in [η5-1,2,4-(Me3C)3C5H2]2UMe2 (2.345(11) and 2.399(8) Å)8a but comparable to those observed in {[(Me3SiNCH2CH2)3N]U[μ-N(SiMe3)NC]}2 (2.540(2) Å)10c and [η5-1,3-(Me3Si)2C5H3]2U(bipy)(2,6-Me2PhNC) (2.582(6) Å),12 consistent with a dative coordination of the carbon atom to the U atom. In addition, the N(1)−N(2) (1.300(6) Å) and N(3)−N(4) (1.284(6) Å) distances are elongated compared to that in free diazoalkanes (1.12–1.13 Å),13 but similar to those found in diazoalkyl compounds [(2-iPr2P-4-MePh)2N]2U=N2CPh2 (1.359(7) Å),11a (η5-C5Me5)2U(=N2CPh2)2 (1.315(6) and 1.324(5) Å),11a [iPrNC(NiPr2)NiPr]3U=N2CPh2 (1.299(4) Å),11b and [η5-1,2,4-(Me3Si)3C5H2]2U(bipy)(=N2CHSiMe3) (1.332(6) Å).11c Moreover, the C(57)−N(2) and C(58)-N(4) distances of 1.169(8) Å and 1.172(7) Å, respectively, are identical within the 3σ-criterion. These structural parameters are consistent with the structure C (Scheme 1). Furthermore, the N(1)−N(2)−C(57) and N(3)−N(4)−C(58) angles are close to linearity with 177.9(6) and 179.0(6)°, respectively, whereas the U(1)−N(1)−N(2), U(2)−N(3)−N(4) and U(1)−C(58)−N(4) angles are slightly smaller with 175.9(4), 168.7(4) and 167.9(4)°, respectively.

Synthesis of complex 2.
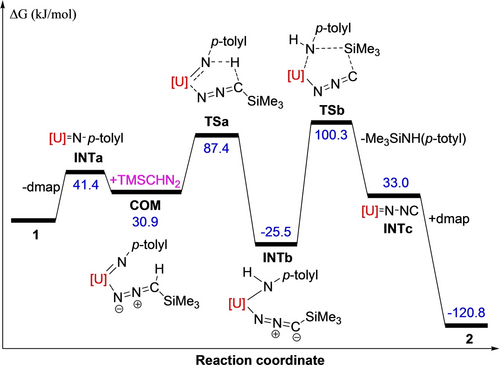
Energy profile (kJ/mol) for the reaction of 1+Me3SiCHN2 (computed at T=353 K). [U]=[η5-1,2,4-(Me3Si)3C5H2]2U.
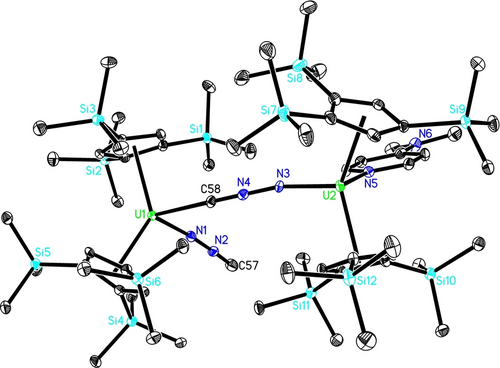
Molecular structure of 2 (thermal ellipsoids drawn at the 35 % probability level).
compound |
C(Cp)−U[b] |
C(Cp)−U[c] |
Cp(cent)−U |
U−X |
Cp(cent)−U−Cp(cent) |
X−U−X/Y |
|---|---|---|---|---|---|---|
2 |
U(1) 2.788(15) U(2) 2.786(15) |
U(1) 2.746(5) to 2.866(5) U(2) 2.732(5) to 2.874(5) |
U(1) 2.500(5), 2.520(5) U(2) 2.518(5), 2.496(5) |
U(1) N(1) 2.035(4), C(58) 2.548(5) U(2) N(3) 2.067(4), N(5) 2.481(5) |
U(1) 132.8(2) U(2) 131.1(2) |
U(1) 88.6(2) U(2) 92.6(2) |
5 |
U(1) 2.73(1) U(2) 2.74(2) U(3) 2.75(1) |
U(1) 2.68(3) to 2.79(2) U(2) 2.61(2) to 2.83(2) U(3) 2.67(2) to 2.78(2) |
U(1) 2.45(2), 2.45(2) U(2) 2.47(2), 2.48(2) U(3) 2.47(2), 2.48(2) |
U(1) N(6A) 2.130(18), C(27) 2.48(3) U(2) N(2) 2.086(19), C(54) 2.43(2) U(3) N(4) 2.143(19), C(81) 2.380(17) |
U(1) 136.0(6) U(2) 133.2(7) U(3) 134.2(7) |
U(1) 105.5(7) U(2) 98.1(7) U(3) 102.7(7) |
6 |
2.721(18) |
2.655(7) to 2.767(8) |
2.445(7), 2.442(7) |
I(1) 2.986(1), I(2) 2.936(1) |
127.8(2) |
101.2(1) |
7 |
2.808(26) |
2.724(9) to 2.871(9) |
2.541(9), 2.541(9) |
N(1) 2.388(8), N(2) 2.646(8) N(1A) 2.388(8), N(2A) 2.646(8) |
111.5(3) |
145.2(4)[d] |
- [a] Cp=cyclopentadienyl ring. [b] average value, the value in parentheses is standard deviation of the mean. [c] Range. [d] The angle of N(1)−U(1)−N(1A).
In contrast, the less sterically hindered the uranium imido metallocene [η5-1,3-(Me3C)2C5H3]2U=N(p-tolyl)(dmap) (4), derived from [η5-1,3-(Me3C)2C5H3]2UMe2 (3) and p-tolylNH2 in the presence of dmap, reacts with Me3SiCHN2 in toluene at 50 °C to form a different product. Instead of a bimetallic complex, the hexametallic isocyanoimido uranium cluster {[η5-1,3-(Me3C)2C5H3]2U[μ-(=NNC)]}6 (5) is isolated in good yield (Scheme 3). This difference is attributed to the reduced steric hindrance at the uranium atom in 4. Figure 3 shows the molecular structure of 5. Each [η5-1,3-(Me3C)2C5H3]2U fragment coordinates κN to to the terminal nitrogen atom of a [NNC]2− group and κC to the carbon atom of another [CNN]2− group, resulting in a self-assembly of six {[η5-1,3-(Me3C)2C5H3]2U}2+ cations and six [NNC]2− anions to form the hexameric macro-ring14 with U(1)−U(3A), U(1)−U(2) and U(2)−U(3) distances of 6.933(1), 6.935(1) and 6.941(1) Å, respectively. The coordination environment at the uranium atom is best described as distorted-tetrahedral. The U(1)−N(6A), U(2)−N(2) and U(3)−N(4) distances are 2.130(18), 2.086(19) and 2.143(19) Å (Table 1), respectively, whereas the U(1)−C(27), U(2)−C(54) and U(3)−C(81) distances are 2.48(3), 2.43(2) and 2.380(17) Å, respectively. Overall, these structural parameters are comparable to those observed in complex 2. Furthermore, complexes 2 and 5 exhibit the ν(C=N=N) stretching frequencies at 2117 cm−1 and 1990 cm−1, respectively, which are in line with those (1950–2035 cm−1) detected in other diazoalkyl complexes.5b
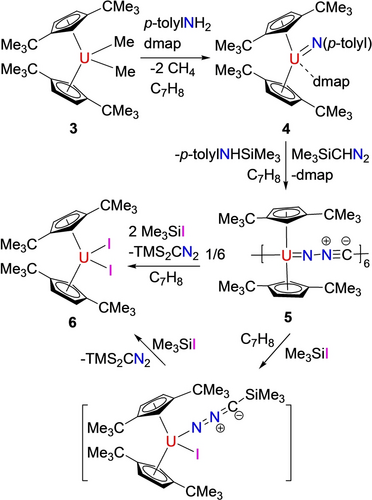
Synthesis of complexes 4–6.
Molecular structure of 5 (tert-butyl group omitted for clarity, thermal ellipsoids drawn at the 35 % probability level).
Bonding Studies
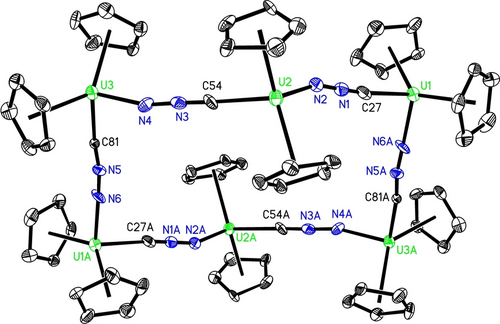
Density functional theory (DFT) computations at the B3PW91 level of theory were performed on the model complex [η5-1,3-(Me3C)2C5H3]2U(=NNC) (5′) to evaluate the bonding between the [η5-1,3-(Me3C)2C5H3]2U2+ and [NNC]2− fragments. These studies identified one U−N σ-bond and two U−N π-bonds as illustrated in Figure 4. The natural bond orbital (NBO) analysis reveals that U−N σ-bond, σ(U=N), is formed by a nitrogen hybrid lone pair (63.8 % 2 s and 36.2 % 2p) donating to a vacant U orbital. The two orthogonal U−N π bonds, π1 and π2, have similar compositions and consist of a pure 2p nitrogen-based orbital (78.3 %) and a uranium hybrid orbital (21.7 %; 1.7 % 7p+52.6 % 6d+45.7 % 5f) and a pure 2p nitrogen-based orbital (74.4 %) and a uranium hybrid orbital (25.6 %; 0.4 % 7p+25.7 % 6d+73.9 % 5f), respectively. These values are comparable to those computed for the imido metallocene (η5-C5H5)2U=NMe.15a Within this description additional electron density is transferred from the π-orbitals of [NNC]2− fragment to the electron deficient metallocene unit [η5-1,3-(Me3C)2C5H3]2U2+. Moreover, the calculated natural charges for U, Nα, Nβ and Cγ are 1.44, −0.72, −0.28 and 0.08, respectively, whereas the averaged natural charge for the two 1,3-(Me3C)2C5H3 ligands is −0.26. The Wiberg bond orders are 1.51 for U−Nα, 1.17 for Nα-Nβ, and 2.32 for Nβ-Cγ, respectively, consistent with an isocyanidoimido resonance structure (Scheme 1, C). Based on these computations a significant involvement of the uranium 5 f and 6d orbitals in the bonding between the metallocene [η5-1,3-(Me3C)2C5H3]2U2+ and [NNC]2− fragment can be assumed as previously shown in imido metallocene (η5-C5H5)2U=NMe,15a indicating the 5 f as well as the 6d orbitals participate in the bonding of actinide complexes and that the U=N bonds are generally more polarized than those in related d-transition metals.15b Furthermore, a multiconfigurational, CASSCF computation on the geometry optimized at the DFT level of theory gives a triplet electronic ground state comparable to that found in (η5-C5H5)2U=NMe.15a Similar to (η5-C5H5)2U=NMe15a the triplet ground state is composed of two determinants (85 % and 15 %), indicating that this state is properly described by a single reference configuration.15a Moreover, the geometry optimization at the CASSCF level gives a geometry identical to that obtained at the DFT level of theory, further validating the use of DFT methods for [η5-1,3-(Me3C)2C5H3]2U(=NNC) (5′).
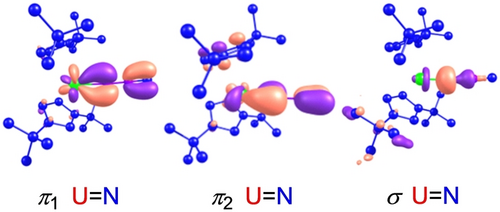
Plots of HOMOs for 5′ (the hydrogen atoms have been omitted for clarity).
Alternatively, the bonding in complex 5′ may also be analyzed using the quantum theory of atoms in molecules (QTAIM), which is based on the topology of the electron density at a bond critical point (BCP).9 QTAIM data (ρb=0.156, Hb=−0.078) are in line with a polarized U+−N− bond, but also possesses covalent character. Moreover, the calculated delocalization indices (DI=2.29) indicate significantly accumulated electron density in the U−N bonding region of the complex 5′. Hence, the QTAIM and NBO analyses arrive at similar conclusions.
Reactivity Studies
The polarized nature of the U=NNC moiety may also impact its reactivity, and it is instructive to compare this reactivity to that of the imido compound [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (1).9 The bimetallic isocyanoimido uranium complex 2 is surprisingly stable and has so far proven to be inert to a series of small molecules such as Me3SiX (X=Cl, I), CS2 and RC≡CR (R=Ph, Me) even when heated to 100 °C for one week. This lack of reactivity is presumably a consequence of the effective shielding of the uranium atoms by the Cp ligands hindering the substrate approach to the uranium atom.
In contrast, treatment of 5 with Me3SiI gives the diiodido complex [η5-1,3-(Me3C)2C5H3]2UI2 (6) concomitant with (Me3Si)2CN2 loss (Scheme 3). Most likely, this reaction proceeds via a monoiodido intermediate [η5-1,3-(Me3C)2C5H3]2U(NNCSiMe3)(I) but it readily converts in the presence of a second molecule of Me3SiI to yield 6 and (Me3Si)2CN2 (Scheme 3). The U4+ atom in 6 is η5-bound to two eclipsed Cp-rings and coordinates to two iodide ligands in a distorted-tetrahedral fashion (Figure 5) with an average U−C(ring) distance of 2.721(18) Å, the Cp(cent)−U−Cp(cent) angle of 127.8(2)° and the I−U−I angle of 101.2(1)° (Table 1). The U−I(1) and U−I(2) distances are rather asymmetric with 2.986(1) and 2.936(1) Å, respectively, but they are comparable to those found in (η5-C5Me5)2UI2 (2.981(1) Å and 2.987(1) Å),16a (η5-C5iPr4H)2UI2 (2.964(1) Å and 2.973(1) Å),16b [η5-C5(CH2Ph)5]2UI2 (2.962(1) Å and 2.948(1) Å),16c and [η5-1,2,4-(Me3Si)3C5H2]2UI2 (2.966(1) Å and 2.952(1) Å).11c
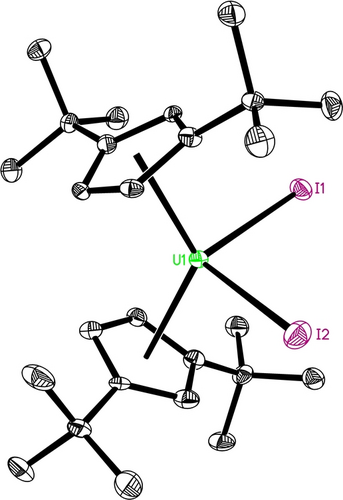
Molecular structure of 6 (thermal ellipsoids drawn at the 35 % probability level).
Moreover, like the uranium imido compound [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (1),9 complex 5 is reactive toward hetero-unsaturated organic substrates. For example, addition of a toluene solution of CS2 to 5 in the presence of dmap forms the bis(isothiocyanate) uranium metallocene, [η5-1,3-(Me3C)2C5H3]2U(NCS)2(dmap)2 (7) in good yield (Scheme 4).
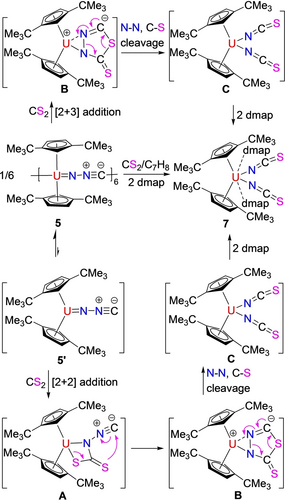
Synthesis of complex 7.
We propose that under these conditions an initial dissociation of 5 to form monomeric intermediate [η5-1,3-(Me3C)2C5H3]2U(=NNC) (5′) occurs, followed by a reaction with CS2 proceeding via a [2+2] cycloaddition to give a four-membered intermediate A (Scheme 4; down), which then converts to the thioadiazolonediide intermediate B. In a next step, intermediate B undergoes N−N and C−S bond cleavage to yield a bis-isothiocyanate complex C, that rapidly reacts with dmap to give the final product 7 (Scheme 4; down). Alternatively, complex 5 (5′) may directly convert with CS2 via a [2+3] cycloaddition to give the thioadiazolonediide intermediate B (Scheme 4; up), and cleavage of the N−N and C−S bonds result in the bis-isothiocyanate complex C, which then forms adduct 7 in the presence of dmap (Scheme 4; up). To differentiate both pathways DFT computations were performed revealing that in contrast to the reactivity of the diazomethanediide-bridged platinum(II) complex [(tBu2PCHCH)2NPt]2(μ-κN : κC-CNN) with CO2,7 a direct approach of CS2 to the [U=NNC] moiety in [η5-1,3-(Me3C)2C5H3]2U(=NNC) (5′) does not initiate a [2+3] cycloaddition, instead, complex 5′ initially engages in a [2+2] cycloaddition with CS2 to give a four-membered intermediate INT7a (Figure 6), which then converts to the thioadiazolonediide intermediate INT7b. In a next step, intermediate INT7b isomerizes barrierless to INT7c, that undergoes simultaneous N−N and C−S cleavage to yield the bis-isothiocyanate INT7d, which forms 7 in the presence of dmap. The formation of 7 is energetically extremely favorable (ΔG(298 K)=−513.3 kJ mol−1) and occurs with a low activation barrier of only ΔG≠(298 K)=7.1 kJ mol−1 (Figure 6). The U4+ atom in 7 is η5-bound to two eclipsed Cp-rings and κN-coordinated to four nitrogen atoms to form a distorted-octahedral coordination sphere (Figure 7) with an average U−C(ring) distance of 2.808(26) Å, the Cp(cent)−U−Cp(cent) angle of 111.5(3)° and the N(1)−U−N(1A) angle of 145.2(4)° (Table 1). The U−N(1) distance to the ionic isothiocyanate ligands is 2.388(8) Å, whereas the U−N(2) distance to the neutral dmap ligands is much longer with 2.646(8) Å. Moreover, the N(1)−C(14) and C(14)−S(1) distances are 1.171(12) and 1.611(11) Å, respectively, and the angles of U(1)−N(1)−C(14) and N(1)−C(14)−S(1) are 172.2(8) and 177.6(9)°, respectively. Furthermore, complex 7 exhibits the ν(N=C=S) stretching frequency at 2021 cm−1, which is in line with those (1980–2200 cm−1) established in isothiocyanate complexes,17a and comparable to that (2047 cm−1) found in [Bu4N]4[U(NCS)8].17b However, in contrast to the imido [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (1),9b no reaction occurs between complex 5 and internal alkynes RC≡CR or RC≡CC≡CR (R=Ph, Me) even when heated to 100 °C for one week, presumably due to the more polarized nature of the uranium-isocyanoimido bond U+-[NNC]−.15b
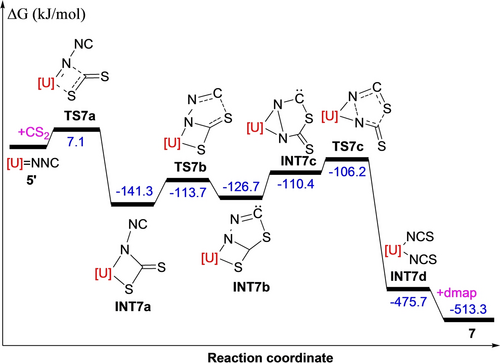
Energy profile (kJ/mol) for the reaction of 5′+CS2 (computed at T=298 K). [U]=[η5-1,3-(Me3C)2C5H3]2U.
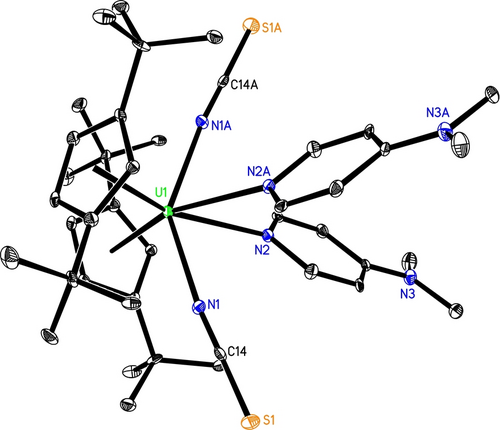
Molecular structure of 7 (thermal ellipsoids drawn at the 35 % probability level).
Conclusion
In conclusion, the first isocyanoimido complexes, [η5-1,2,4-(Me3Si)3C5H2]2U(=NNC)(μ-CNN=)U(dmap)[η5-1,2,4-(Me3Si)3C5H2]2 (2) and {[η5-1,3-(Me3C)2C5H3]2U[μ-(=NNC)]}6 (5), were successfully prepared and they exhibit remarkable thermal stability in the solid-state decomposing above 230 °C and 265 °C, respectively. This suggests that other stable metal isocyanoimido complexes may also be accessible, provided a viable synthetic route is available. DFT computations imply that the 5f and 6d orbitals contribute significantly to the bonding within the uranium isocyanoimido U=NNC moiety, resulting in the very polarized bonds between the Cp2U2+ and [NNC]2− fragments. Hence, complex 5 reacts as a nucleophile in the presence of alkylsilyl halides and participates in a [2+2] cycloaddition with CS2, but it shows no reactivity with internal alkynes. The latter aspect may be attributed to the rather polarized nature of the uranium-isocyanoimido bond U+-[NNC]−.15b This study also illustrates how small variations in the supporting cyclopentadienyl ligand effect the structure and reactivity of these uranium complexes. For example, reaction of the uranium imido complex [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (1) with Me3SiCHN2 forms the dimeric species [η5-1,2,4-(Me3Si)3C5H2]2U(=NNC)(μ-CNN=)U(dmap)[η5-1,2,4-(Me3Si)3C5H2]2 (2), whereas the less-sterically demanding imido compound [η5-1,3-(Me3C)2C5H3]2U=N(p-tolyl)(dmap) (4) affords a hexameric complex {[η5-1,3-(Me3C)2C5H3]2U[μ-(=NNC)]}6 (5). The Cp ligands not only dictate different structures, but also affect the reactivity of these species. While 5 is a reactive molecule, 2 has shown no reactivity in small molecule activation so far. The development of new diazomethanediide complexes containing a terminal multiple-bonded isocyanoimido moiety (M=NNC) (Scheme 1, C) or a diazocarbene (M=CN2) ligand (Scheme 1, D) and exploration of their reactivity are ongoing and will be communicated in due course.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 22271017), and the Deutsche Forschungsgemeinschaft (DFG) through the Heisenberg program (WA 2513/8). Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.