Atroposelective Synthesis of C−N Vinylindole Atropisomers by Palladium-Catalyzed Asymmetric Hydroarylation of 1-Alkynylindoles
Li-Wen Zhan
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Chuan-Jun Lu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Jia Feng
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ren-Rong Liu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorLi-Wen Zhan
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Chuan-Jun Lu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Jia Feng
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ren-Rong Liu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorGraphical Abstract
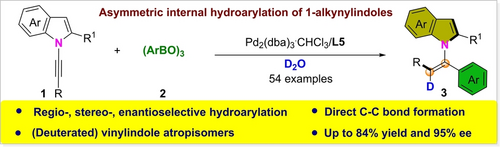
The enantioselective hydroarylation of 1-alkynylindoles with organoborons for the synthesis of chiral C−N atropisomers is presented. A wide variety of vinylindole atropisomers were synthesized in excellent regioselectivity, stereoselectivity (Z-selectivity), and enantioselectivity under mild reaction conditions.
Abstract
Transition-metal-catalyzed hydroarylation of unsymmetrical internal alkynes remains challenging because of the difficulty in controlling regioselectivity and stereoselectivity. Moreover, the enantioselective hydroarylation of alkynes using organoboron reagents has not been reported. Herein, we report for the first time that palladium compounds can catalyze the hydroarylation of 1-alkynylindoles with organoborons for the synthesis of chiral C−N atropisomers. A series of rarely reported vinylindole atropisomers was synthesized with excellent regio-, stereo- (Z-selectivity), and enantioselectivity under mild reaction conditions. The ready availability of organoborons and alkynes and the simplicity, high stereoselectivity, and good functional group tolerance of this catalytic system make it highly attractive.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202312930-sup-0001-misc_information.pdf26 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Bringmann, S. Tasler, H. Endress, J. Kraus, K. Messer, M. Wohlfarth, W. Lobin, J. Am. Chem. Soc. 2001, 123, 2703–2711;
- 1bC. Ito, Y. Thoyama, M. Omura, I. Kajiura, H. Furukawa, Chem. Pharm. Bull. 1993, 41, 2096–2100;
- 1cR. S. Norton, R. J. Wells, J. Am. Chem. Soc. 1982, 104, 3628–3635;
- 1dC. C. Hughes, A. Prieto-Davo, P. R. Jensen, W. Fenical, Org. Lett. 2008, 10, 629–631;
- 1eG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563–639;
- 1fS. T. Toenjes, J. L. Gustafson, Future Med. Chem. 2018, 10, 409–422;
- 1gJ. E. Smyth, N. M. Butler, P. A. Keller, Nat. Prod. Rep. 2015, 32, 1562–1583;
- 1hG.-J. Mei, W. L. Koay, C.-Y. Guan, Y. Lu, Chem 2022, 8, 1855–1893.
- 2For reviews, see:
- 2aT. Z. Li, S. J. Liu, W. Tan, F. Shi, Chem. Eur. J. 2020, 26, 15779–15792;
- 2bY.-C. Zhang, F. Jiang, F. Shi, Acc. Chem. Res. 2020, 53, 425–446;
- 2cJ. K. Cheng, S.-H. Xiang, S. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805–4902;
- 2dO. Kitagawa, Acc. Chem. Res. 2021, 54, 719–730;
- 2eF. Colobert, B.-F. Shi, Chem Catal. 2021, 1, 483–485;
- 2fY.-J. Wu, G. Liao, B.-F. Shi, Green Synth. Catal. 2022, 3, 117–136;
- 2gP. Rodríguez-Salamanca, R. Fernández, V. Hornillos, J. M. Lassaletta, Chem. Eur. J. 2022, 28, e202104442;
- 2hH.-H. Zhang, F. Shi, Acc. Chem. Res. 2022, 55, 2562–2580;
- 2iB.-M. Yang, X. Q. Ng, Y. Zhao, Chem Catal. 2022, 2, 3048–3076. For selected examples, see:
- 2jY. Morimoto, S. Shimizu, A. Mokuya, N. Ototake, A. Saito, O. Kitagawa, Tetrahedron 2016, 72, 5221–5229;
- 2kL. Wang, J. Zhong, X. Lin, Angew. Chem. Int. Ed. 2019, 58, 15824–15828;
- 2lW. Xia, Q.-J. An, S.-H. Xiang, S. Li, Y.-B. Wang, B. Tan, Angew. Chem. Int. Ed. 2020, 59, 6775–6779;
- 2mJ. Frey, A. Malekafzali, I. Delso, S. Choppin, F. Colobert, J. Wencel-Delord, Angew. Chem. Int. Ed. 2020, 59, 8844–8848;
- 2nQ. Ren, T. Cao, C. He, M. Yang, H. Liu, L. Wang, ACS Catal. 2021, 11, 6135–6140;
- 2oA. Kim, A. Kim, S. Park, S. Kim, H. Jo, K. M. Ok, S. K. Lee, J. Song, Y. Kwon, Angew. Chem. Int. Ed. 2021, 60, 12279–12283;
- 2pL. Sun, H. Chen, B. Liu, J. Chang, L. Kong, F. Wang, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2021, 60, 8391–8395;
- 2qV. Corti, M. K. Thøgersen, V. J. Enemærke, N. M. Rezayee, C. L. Barløse, K. A. Jørgensen, Chem. Eur. J. 2022, 28, e202202395;
- 2rK.-W. Chen, Z.-H. Chen, S. Yang, S.-F. Wu, Y.-C. Zhang, F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202116829;
- 2sY. Gao, L.-Y. Wang, T. Zhang, B.-M. Yang, Y. Zhao, Angew. Chem. Int. Ed. 2022, 61, e202200371;
- 2tP. Zhang, Q. Xu, X.-M. Wang, J. Feng, C.-J. Lu, Y. Li, R.-R. Liu, Angew. Chem. Int. Ed. 2022, 61, e202212101;
- 2uZ.-S. Wang, L.-J. Zhu, C.-T. Li, B.-Y. Liu, X. Hong, L.-W. Ye, Angew. Chem. Int. Ed. 2022, 61, e202201436;
- 2vR. R. Surgenor, X. Liu, M. J. H. Keenlyside, W. Myers, M. D. Smith, Nat. Chem. 2023, 15, 357–365;
- 2wZ.-H. Chen, T.-Z. Li, N.-Y. Wang, X.-F. Ma, S.-F. Ni, Y.-C. Zhang, F. Shi, Angew. Chem. Int. Ed. 2023, 62, e202300419.
- 3For olefin atropisomeric reviews, see:
- 3aJ. Feng, Z. Gu, SynOpen 2021, 5, 68–85;
- 3bS. Wu, S.-H. Xiang, J. K. Cheng, B. Tan, Tetrahedron Chem 2022, 1, 100009.
10.1016/j.tchem.2022.100009 Google Scholar
- 4
- 4aR. Mi, H. Chen, X. Zhou, N. Li, D. Ji, F. Wang, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2021, 60, e202111860; For a two-step strategy via an allylic substitution/isomerization process, see:
- 4bY.-X. Wu, Q. Liu, Q. Zhang, Z. Ye, Y. He, Cell Reports Phys. Sci. 2022, 3, 101005.
- 5D. Ji, J. Jing, Y. Wang, Z. Qi, F. Wang, X. Zhang, Y. Wang, X. Li, Chem 2022, 8, 3346–3362.
- 6
- 6aY. Yamamoto, Chem. Soc. Rev. 2014, 43, 1575–1600;
- 6bJ. Corpas, P. Mauleón, R. G. Arrayás, J. C. Carretero, ACS Catal. 2021, 11, 7513–7551.
- 7T. Hayashi, K. Inoue, N. Taniguchi, M. Ogasawara, J. Am. Chem. Soc. 2001, 123, 9918–9919.
- 8C. H. Oh, H. H. Jung, K. S. Kim, N. Kim, Angew. Chem. Int. Ed. 2003, 42, 805–808.
- 9
- 9aM. Lautens, M. Yoshida, Org. Lett. 2002, 4, 123–125;
- 9bH. Imase, T. Suda, Y. Shibata, K. Noguchi, M. Hirano, K. Tanaka, Org. Lett. 2009, 11, 1805–1808;
- 9cY. Yasuhara, T. Nishimura, T. Hayashi, Chem. Commun. 2010, 46, 2130–2132;
- 9dJ. Panteleev, R. Y. Huang, E. K. J. Lui, M. Lautens, Org. Lett. 2011, 13, 5314–5317;
- 9eT. Shibuya, Y. Shibata, K. Noguchi, K. Tanaka, Angew. Chem. Int. Ed. 2011, 50, 3963–3967;
- 9fY. Bai, J. Yin, W. Kong, M. Mao, G. Zhu, Chem. Commun. 2013, 49, 7650–7652;
- 9gM. Hoshi, O. Kaneko, M. Nakajima, S. Arai, A. Nishida, Org. Lett. 2014, 16, 768–771;
- 9hS. Arae, S. Beppu, T. Kawatsu, K. Igawa, K. Tomooka, R. Irie, Org. Lett. 2018, 20, 4796–4800;
- 9iJ. Corpas, P. Mauleón, R. G. Arrayás, J. C. Carretero, Org. Lett. 2020, 22, 6473–6478;
- 9jB. M. Trost, J. J. Cregg, C. Hohn, W.-J. Bai, G. Zhang, J. S. Tracy, Nat. Chem. 2020, 12, 629–637.
- 10
- 10aD. Liu, J. Derosa, K. M. Engle, J. Am. Chem. Soc. 2016, 138, 13076–13081;
- 10bL. E. Hanna, M. O. Konev, E. R. Jarvo, Eur. J. Org. Chem. 2019, 184–187;
- 10cA. Qin, H. Qian, Q. Chen, S. Ma, Chin. J. Chem. 2020, 38, 372–382;
- 10dY. Pang, G. Liu, C. Huang, X.-A. Yuan, W. Li, J. A. Xie, Angew. Chem. Int. Ed. 2020, 59, 12789–12794;
- 10eH. Wang, H. Luo, Z.-M. Zhang, W.-F. Zheng, Y. Yin, H. Qian, J. Zhang, S. Ma, J. Am. Chem. Soc. 2020, 142, 9763–9771.
- 11
- 11aT. Miura, M. Shimada, M. Murakami, J. Am. Chem. Soc. 2005, 127, 1094–1095;
- 11bZ. Li, A. García-Domínguez, C. Nevado, J. Am. Chem. Soc. 2015, 137, 11610–11613;
- 11cR. Shintani, S. Isobe, M. Takeda, T. Hayashi, Angew. Chem. Int. Ed. 2010, 49, 3795–3798;
- 11dD. J. Burns, H. W. Lam, Angew. Chem. Int. Ed. 2014, 53, 9931–9935;
- 11eC. Clarke, C. A. Incerti-Pradillos, H. W. Lam, J. Am. Chem. Soc. 2016, 138, 8068–8071;
- 11fM. Callingham, B. M. Partridge, W. Lewis, H. W. Lam, Angew. Chem. Int. Ed. 2017, 56, 16352–16356;
- 11gS. N. Karad, H. Panchal, C. Clarke, W. Lewis, H. W. Lam, Angew. Chem. Int. Ed. 2018, 57, 9122–9125;
- 11hN. Iqbal, D. Maiti, E. J. Cho, Angew. Chem. Int. Ed. 2019, 58, 15808–15812;
- 11iJ. Chen, Y. Wang, Z. Ding, W. Kong, Nat. Commun. 2020, 11, 1882;
- 11jZ. Ding, Y. Wang, W. Liu, Y. Chen, W. Kong, J. Am. Chem. Soc. 2021, 143, 53–59;
- 11kK. Wang, J. Chen, W. Liu, W. Kong, Angew. Chem. Int. Ed. 2022, 61, e202212664.
- 12For a review on the synthesis of atropisomers through catalytic asymmetric reactions of alkynes, see: Z.-X. Zhang, T.-Y. Zhai, L.-W. Ye, Chem Catal. 2021, 1, 1378.
- 13
- 13aF. Xue, T. Hayashi, Angew. Chem. Int. Ed. 2018, 57, 10368–10372; For a combined copper- and palladium-catalyzed atroposelective arylboration of symmetric alkynes, see:
- 13bW. Li, S. Chen, J. Xie, Z. Fan, K. Yang, Q. Song, Nat. Synth. 2023, 2, 140–151.
- 14For application of indole-derived alkenes, see:
- 14aC. C. Sorensen, F. A. Leibfarth, J. Am. Chem. Soc. 2022, 144, 8487–8492;
- 14bL. Li, J. Ren, J. Zhou, X. Wu, Z. Shao, X. Yang, D. Qian, Nat. Commun. 2022, 13, 6861.
- 15Deposition number 2272170 (3 a) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 16The rotational barrier of compound 3 s was measured to be 27.5 kal/mol, which indicated that decrease of 3 s in the ee may be due to the partial thermal racemization of product. Under the standard reaction conditions (65 °C), 3 r: 70 %, 50 % ee.
- 17S. R. LaPlante, L. D. D. D. Fader, K. R. Fandrick, D. R. Fandrick, O. Hucke, R. Kemper, S. P. F. Miller, P. J. Edwards, J. Med. Chem. 2011, 54, 7005–7022.
- 18When the reaction of 1-alkynylindole (1 a) and phenylboroxine (2 a) was performed with 10.0 equivalent D2O, the level of D incorporation can be increased to 80 %, but in this case the enantioselectivity of 3 a was reduced to 87 %.
- 19
- 19aK. Saito, S. Miwa, A. Iida, Y. Fujimoto, E. Caytan, C. Roussel, O. Kitagawa, Org. Lett. 2021, 23, 7492–7496;
- 19bX. Li, G.-W. Wang, L.-X. Liu, C.-B. Yu, Y.-G. Zhou, Angew. Chem. Int. Ed. 2023, 62, e202301337.