Pd Loaded NiCo Hydroxides for Biomass Electrooxidation: Understanding the Synergistic Effect of Proton Deintercalation and Adsorption Kinetics
Graphical Abstract
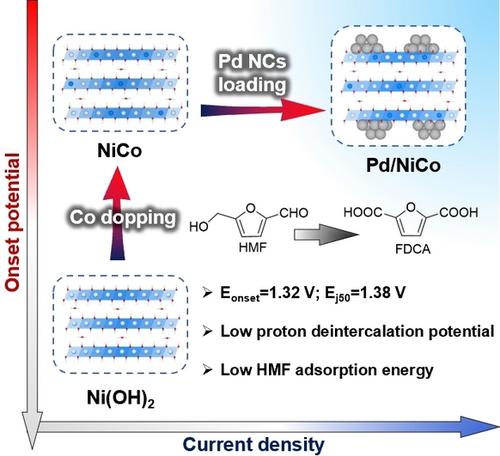
By loading Pd NCs on the Co-doped Ni(OH)2, we were able to promote the proton deintercalation process on the catalyst surface as well as HMF adsorption, respectively. As a result, a comprehensive optimization in performance by simultaneously decreasing the onset potential of the reaction and enhancing the current density can be achieved.
Abstract
The key issue in the 5-hydroxymethylfurfural oxidation reaction (HMFOR) is to understand the synergistic mechanism involving the protons deintercalation of catalyst and the adsorption of the substrate. In this study, a Pd/NiCo catalyst was fabricated by modifying Pd clusters onto a Co-doped Ni(OH)2 support, in which the introduction of Co induced lattice distortion and optimized the energy band structure of Ni sites, while the Pd clusters with an average size of 1.96 nm exhibited electronic interactions with NiCo support, resulting in electron transfer from Pd to Ni sites. The resulting Pd/NiCo exhibited low onset potential of 1.32 V and achieved a current density of 50 mA/cm2 at only 1.38 V. Compared to unmodified Ni(OH)2, the Pd/NiCo achieved an 8.3-fold increase in peak current density. DFT calculations and in situ XAFS revealed that the Co sites affected the conformation and band structure of neighboring Ni sites through CoO6 octahedral distortion, reducing the proton deintercalation potential of Pd/NiCo and promoting the production of Ni3+−O active species accordingly. The involvement of Pd decreased the electronic transfer impedance, and thereby accelerated Ni3+−O formation. Moreover, the Pd clusters enhanced the adsorption of HMF through orbital hybridization, kinetically promoting the contact and reaction of HMF with Ni3+−O.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.