Dinor[7]helicene and Beyond: Divergent Synthesis of Chiral Diradicaloids with Variable Open-Shell Character
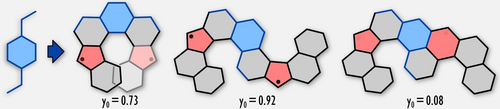
Graphical Abstract
One starting material, two ring-forming steps, and three fusion patterns. A sequence of electrophilic cyclizations enables the first synthesis of dinor[7]helicene, a configurationally stable, redox-active diradicaloid analogue of [7]helicene. Strain-induced rearrangements of larger aromatic precursors provide access to non-benzenoid bis-helicenes, whose open-shell character depends on the connectivity between spin centers.
Abstract
Diradicaloid helicenes constructed formally by non-benzenoid double π-extension of phenanthrene were synthesized by a common strategy involving double electrophilic benzannulation. Steric effects in the second benzannulation step led to considerable structural diversity among the products, yielding a symmetrical dinor[7]helicene 1 and two isomeric unsymmetrical double helicenes 2 and 3, containing a nor[5]helicene and [4]helicene fragment, respectively, in addition to a common nor[6]helicene motif. Geometries, configurational dynamics, and electronic structure of these helicenes were analyzed using solid-state structures, spectroscopic methods, and computational analyses. The open-shell character of the singlet states of these helicenes increases in the order 3<1<2, with strongly varying diradicaloid indexes and singlet–triplet gaps. Compounds 1–3 displayed narrow optical gaps of 0.79–1.25 eV, resulting in significant absorption in the near infrared (NIR) region. They also exhibit reversible redox chemistry, each of them yielding stable radical cations, radical anions, and dianions, in some cases possessing intense NIR absorptions extending beyond 2500 nm.
Introduction
Organic diradicals and diradicaloids combine several desirable characteristics, including temperature-dependent paramagnetism, small HOMO–LUMO gaps, and reversible redox chemistry. Consequently, such compounds have been of interest as near-infrared (NIR) dyes, materials for organic photodiodes, semiconductors for field-effect transistors, and two-photon absorbers.1 However, the inherently high reactivity of diradicals and, to a lesser extent, diradicaloids presents a challenge in molecular design and synthesis. Under ambient conditions, the stability of these open-shell organics is often limited, e.g., by their susceptibility to polymerization and reactions with atmospheric oxygen. This problem is typically addressed by extending the π-electron system of the diradicaloid motif or by the use of bulky blocking groups at positions with high spin density. Examples of both strategies are provided by the family of diindenoarene diradicaloids,2-4 which are typically based on polycyclic aromatic cores,5 including benzenoid hydrocarbons6-21 and heteroaromatic motifs22, 23 (Figure 1).
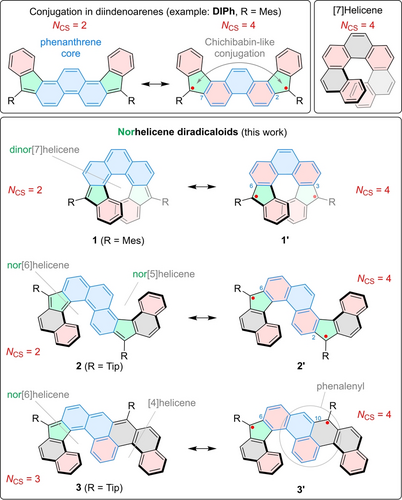
Norhelicene diradicaloids derived from phenanthrene. Phenanthrene moieties, five-membered (“nor-”) rings, and Clar sextets are shaded in blue, green, and red, respectively. Mes, mesityl; Tip, 2,4,6-triisopropylphenyl; NCS, Clar-sextet count.
Incorporation of diradicaloid units into helicene structures may be envisaged as a complementary approach toward stable open-shell aromatics, relying on the overlaps inside the coiled π-systems of helicenes for steric protection. Additionally, the chirality of higher helicenes may lead to optically active diradicals, displaying e.g., strong circular dichroism responses at long wavelengths. In spite of these possibilities and the recent progress in chiral radical chemistry,24 access to configurationally stable diradicaloid helicenes is still limited by the available synthetic methodology. Among the reported systems, [5]helicene-based systems25-27 such as cethrenes25, 26 are of particular interest because of their ability to photoswitch between open- and closed-shell structures. Wu's diindeno-fused picene and its analogues28-30 provide notable examples of higher helicenes, in which the open-shell character is strongly influenced by the degree of helix-induced backbone twist. A range of open-shell systems with smaller helical overlaps have been described, including diradicaloid multi-helicenes16, 31, 32 and a recently reported mono/triradical.33
In this work, we describe a concise synthesis of the diradicaloid 1 (Figure 1), which contains an ortho-fused sequence of 5- and 6-membered rings. Compound 1 can be seen as a pristine analogue of [7]helicene34 with no additional fused rings, and can be called dinor[7]helicene to indicate the two missing methine units.35 The structure of 1 can also be described as a diindenophenanthrene, with the formal radical centers adjacent to positions 3 and 6 of the phenanthrene core (cf. 1′). In the previously reported diindenophenanthrene isomers12, 13 such as DIPh (Figure 1), the radical centers were adjacent to phenanthrene positions 2 and 7, leading to a spin coupling pathway analogous to that of the Chichibabin hydrocarbon.36, 37 The different connectivity of radical sites in 1 is expected to significantly affect the electronic properties of the system, similarly as observed for indenofluorene isomers.38-42 When an attempt was made to prepare an extended, [9]helicene analog of 1, the present method produced instead unprecedented bishelicenes 2 and 3, each containing non-equivalent radical sites. While 2 is an extended diindenophenanthrene with a 2,6 attachment pattern at the phenanthrene, compound 3 features a 3,9 attachment pattern and a unique combination of indene- and phenalenyl-like environments for the two radical centers. Structural differences among helicenes 1–3 lead to variation of their open-shell character, with a major impact on their magnetic, optical, and redox properties.
Results and Discussion
Syntheses of higher helicenes require a strain-inducing step capable of building helical curvature into the aromatic framework.43, 44 In our approach to dinor[7]helicene 1, early introduction of strain was considered advantageous, in view of the potentially low stability of this open-shell target. 1 was therefore disconnected into the highly twisted 4,5-di-o-tolylphenanthrene 6, which contains the complete inner edge of the helicene (Scheme 1). 6 is accessible via a double electrophilic benzannulation involving 1-ethynyl-2-methylbenzene 4 and dialdehyde 5, as recently reported by Itami and co-workers.45, 46 Compound 6 was subjected to radical bromination and subsequent hydrolysis, to yield the key dialdehyde 7, which formed as a mixture of atropisomers. This mixture underwent Grignard addition with mesitylmagnesium bromide, followed by Lewis acid-catalyzed cyclization, to provide the dihydrodinor[7]helicene 8. The final dehydrogenation step was not successful under the standard DDQ oxidation conditions,12 where the dominant (R,R,P)/(S,S,M) diastereomer of 8 proved to be too unreactive. Instead, deprotonation of 8 with KOt-Bu followed by oxidation with CuI provided 1 rapidly and with a high yield.
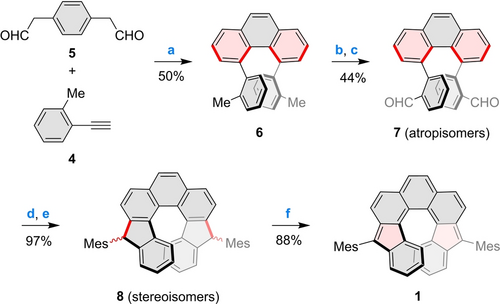
Synthesis of dinor[7]helicene 1. Newly formed bonds and rings are highlighted in red. Reagents and conditions: a) B(C6F5)3, 3 Å molecular sieves, DCM, rt, overnight; b) NBS, CaCO3, hν (470 nm LED), DCM, 8 h; c) CaCO3, H2O/DMF, overnight; d) MesMgBr, THF, rt, overnight; e) BF3⋅Et2O, DCM, rt, 30 min; f) KOt-Bu, CuI, THF, rt, 40 min.
The synthesis of the more extended double helicenes 2 and 3 began with the double condensation between 5 and the bulkier naphthyl-substituted acetylene 9 (Scheme 2). This reaction, carried out using Brønsted acid catalysis, did not produce the highly congested 4,5-diarylphenanthrene 10, furnishing instead its rearranged, 1,5-disubstituted isomer 11.
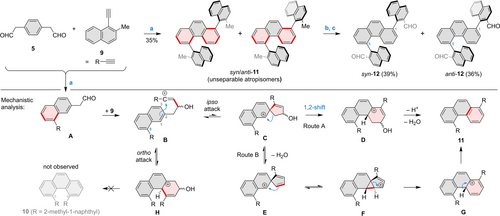
Synthesis of congested diarylphenanthrenes via electrophilic substituent transposition. Reagents and conditions: a) HBF4⋅Et2O, MeNO2, −25 °C, 2 h; b) NBS, CaCO3, hν (470 nm LED), DCM, 1.4 h; c) CaCO3, H2O/DMF, overnight.
The mechanism of this rearrangement was explored using computational methods, which suggested two complementary pathways summarized in Scheme 2 (for complete data, see Scheme S2 and Table S5). In each case, the sequence starts with a singly annulated intermediate A, which produces the 3-hydroxybut-1-en-1-ylium cation B on reaction with another equivalent of 9.47 Subsequent cyclization of B is apparently reversible, yielding the ortho-attack product H as the dominant intermediate. Further dehydration and deprotonation of H, which would lead to the 4,5-diaryl product 10, are apparently kinetically hindered for steric reasons. This hindrance enables the isomerization sequence, starting with the spiro cation C, which forms via an electrophilic ipso attack. C can then undergo ring expansion to cation D via a 1,2-alkyl shift (route A), yielding ultimately the 1,5-diaryl product 11 via dehydration and deprotonation. An alternative course of events, with a slightly lower overall energy barrier (Route B), begins with dehydration of C into E. The latter intermediate can further equilibrate with its tricyclic isomer F, which corresponds to an actual energy minimum and is only slightly more energetic than E. The unusual spiro-fused cyclopropane motif in F is found in the naturally occurring cubebenes,48 and has been invoked in some other carbocation rearrangements.49 In the present case, ring expansion of F is predicted to be non-reversible, yielding the protonated phenanthrene G, and, finally, compound 11.
The non-equivalence of methyl groups in 11 makes it of particular interest as a scaffold for development of unsymmetrical helicenes. Compound 11 was thus transformed into the dialdehyde 12, which formed as a nearly 1 : 1 mixture of syn and anti atropisomers, which were configurationally stable and easily separable by chromatography (Scheme 2). syn-12 and anti-12 were individually subjected to the Grignard addition–cyclization sequence, using TipMgBr as the aryl source (Tip=2,4,6-triisopropylphenyl). In each case, the resulting mixture was found to contain the regioisomeric helicene precursors 13 and 14, originating from competing cyclizations at positions 2 and 10, respectively. The mixtures of 13 and 14 contained several stereoisomers of each product and could not be separated using chromatographic methods. They were thus directly subjected to dehydrogenation under the conditions established for 1, yielding the desired helicenes 2 and 3.
While not separable by standard chromatography, these products were readily isolated in pure form by fractional crystallization, with 2 being much less soluble in MeCN. Different ratios of 2 and 3 were obtained starting from the two atropisomers of 12, with a larger proportion of 3 observed when anti-12 was used (Scheme 3). This difference is attributed to the steric clash that occurs during the cyclization step between the bulky Tip group and the opposite helicene blade or distal ring of the naphthyl group (Scheme S1). The formation of 3 is apparently induced by the bulkiness of the Tip substituents: when the smaller mesityl groups were installed on syn-12, the cyclization occurred nearly exclusively at the phenanthrene position 2, providing 15 as a nearly pure (R,R,P)/(S,S,M) diastereomer, assigned using nuclear Overhauser effect spectroscopy (NOESY, Figure S4). Compound 15 was not useful for diradicaloid synthesis (vide infra), but it provided stereochemical basis for distinguishing the atropisomers of 12.
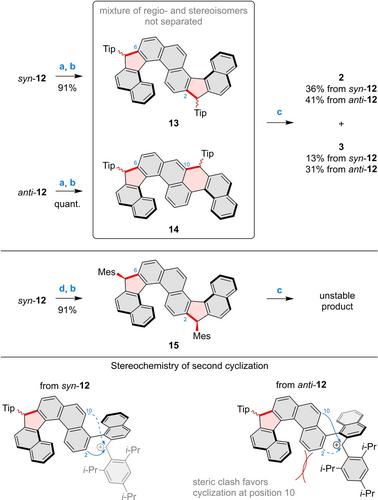
Synthesis of the double helicenes 2 and 3. Reagents and conditions: a) TipMgBr, THF, rt, overnight; b) BF3⋅Et2O, DCM, rt, 30 min; c) KOt-Bu, CuI, THF, rt, 40 min; d) MesMgBr, THF, rt, overnight.
Compounds 1–3 proved to be sufficiently stable, especially in the solid state, to be handled under ambient conditions, although inert atmosphere was used for prolonged storage. In the case of 2, the triisopropylphenyl groups were found to be necessary for achieving sufficient stability. A mesityl-substituted analog of 2, obtained by dehydrogenation of 15, persisted in dilute solutions, but decomposed upon evaporation of the solvent. While 3 likely does not require Tip groups for stability, their presence was needed to direct the cyclization selectivity toward the corresponding precursor 14.
Structures of 1–3 were revealed by X-ray diffraction (XRD) analyses of racemic single crystals (Figure 2).50 The dinor[7]helicene motif in 1 is strongly twisted, with an overall dihedral angle of 51.1° between the terminal benzene rings. Most of the twist is associated with the phenanthrene core, where the dihedral angle along the inner helicene rim is 34.5° in the innermost ring D and decreases toward the termini. Interestingly, the dihedral angles reveal a significant deviation of 1 from the perfect C2 point symmetry, which may be caused by crystal packing forces. The terminal benzene rings A and G partially overlap, with the closest C⋅⋅⋅C distances of 3.1–3.3 Å. In the solid state, relative configurations of the double helicenes 2 and 3 were found to be (P,P)/(M,M) and (P,M)/(M,P), respectively, with the first helicity descriptor corresponding to the nor[6]helicene unit. In both 2 and 3, the nor[6]helicene (rings A−F) had an angle of ca. 47° between the termini and displayed a twisting pattern more evenly distributed among rings C−E. The smaller helical units in these bishelicenes had a much lower overall dihedral angle of 38–40°.
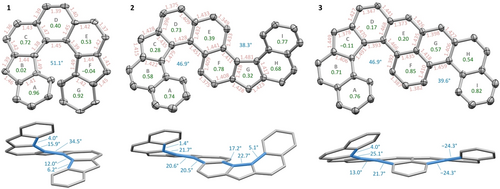
Solid-state structures of 1–3, with ellipsoids drawn at 50 % probability level. Substituents, hydrogen atoms, and solvent molecules are omitted for clarity. One of four symmetry-independent molecules is shown for 1. Bond distances, dihedral angles, and harmonic oscillator model of aromaticity (HOMA) indices51, 52 for rings are shown in red, blue, and green, respectively. Angles between the mean planes of terminal rings in each helical unit are shown in the top view. Side views contain C−C−C−C dihedral angles in the inner helicene rims. Bond length deviations were 0.009-0.010 Å in the structure of 1 and 0.002–0.003 Å in the structures of 2 and 3.
Analysis of bond lengths derived from XRD and density functional theory (DFT) models gave important initial insight into the π-conjugation of 1–3 (Figure 2 and S10). The XRD structure of 3 was very similar to the DFT-optimized singlet geometry (UB3LYP-GD3BJ/6-31 g(d,p)), revealing alternation of bond lengths in rings D and E, along with a negative HOMA51 value in the five-membered ring C (−0.11, XRD data). These features are well described by the closed-shell resonance structure of 3 with a quinodimethane-like bond localization in the D ring, implying a largely closed-shell character. In contrast, 2 shows no significant bond length alternation and relatively high HOMA values in all rings, suggesting a highly open-shell structure. This conjecture is further supported by the similarity of bond length patterns in the singlet and triplet DFT geometries of 2. Finally, an intermediate bonding pattern was determined for dinor[7]helicene 1, with weakly defined quinodimethane localization in rings C and E, and HOMA values close to 0 in the five-membered rings B and F.
Enantiomers of 1–3 proved difficult to separate by enantioselective HPLC, presumably because of the low polarity of our helicenes. Partial resolution was however achieved for 1 and 2, yielding circular dichroism spectra with mirror symmetry (Figure 3). Notably, 1 showed a uniquely strong Cotton effect in the NIR region, with a maximum at ca. 850 nm. The spectra were sufficiently well reproduced by time-dependent DFT calculations to enable assignment of absolute configurations (cf. Figure S19). Enantiomers of 2 racemized with a rate constant krac=9.8 ⋅ 10−4 s−1 at 323 K, corresponding to an extrapolated half-life of 160 min at 298 K. An inversion barrier of ΔG≠,298=22.9(5) kcal/mol was calculated for 2 from the temperature dependence of krac (Figures S22–23).
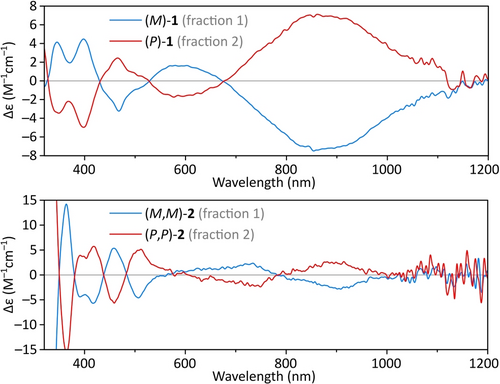
Circular dichroism spectra of enantioenriched samples of 1 and 2. Enantiomer assignment is based on TD-DFT spectra calculated for 11 and 32.
Inversion barriers of the [n]helicene stereogenic units present in 1–3 were estimated computationally at their predicted ground-state multiplicities (11, 32, and 13, see below). In particular, inversion of the dinor[7]helicene unit in 11 proceeded via a Cs-symmetric saddle-shaped transition state TS-7, with a remarkably high ΔG≠,298=47.0 kcal/mol (Figure 4). This barrier is higher than in all-benzenoid [n]helicenes with n≤953 and implies excellent configurational stability of 11 at room temperature. This observation additionally suggests that, even though the all-sp2 five-membered rings contribute a smaller in-plane turn54 to the helicene motif, their greater rigidity results in an overall higher inversion barrier.
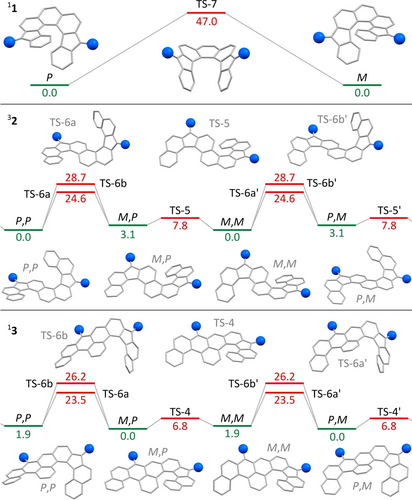
Theoretically predicted isomerization pathways of helicenes 1–3 (UB3LYP-GD3BJ/6-31G(d,p)). Substituents are shown as blue spheres. TS-nx is a transition state involving inversion of a (nor)[n]helicene subunit. Energy minima and transition states are indicated in green and red, respectively. Relative Gibbs free energy values given in kcal/mol.
For the double helicenes 2 and 3, DFT calculations showed that the enantiomeric pairs observed in the solid state, i.e., (P,P)/(M,M)-32 and (P,M)/(M,P)-13, were more stable than the corresponding diastereomers (P,M)/(M,P)-32 and (P,P)/(M,M)-13 by 3.1 and 1.9 kcal/mol, respectively (Figure 4). Inversion of the nor[6]helicene subunits in 32 and 13 occurs via one of two possible transition states, denoted TS-6a and TS-6b. The two states are diastereomeric and differ in the configuration of the saddle-shaped nor[6]helicene moiety relative to the smaller helicene subunit. The corresponding energy barriers (ΔG≠,298=23–25 kcal/mol for the more stable TS-6a) nicely agree with the experimental value obtained for 2, and are also comparable with barriers reported for all-benzenoid [5]helicenes.53, 55 Much lower inversion barriers were determined for the smaller helicene units in 32 (nor[5]helicene, 7.8 kcal/mol) and 13 ([4]helicene, 6.8 kcal/mol), both slightly higher than the computational prediction for [4]helicene.53 Transition state diastereomerism is negligible in these cases, as the geometry of small helicene subunits in TS-4 and TS-5 is close to planar.
Helicenes 1–3 absorb strongly in the visible and near-infrared (NIR) ranges, with absorption onsets above 1000 nm (Figure 5). The apparent optical energy gap of 1 (1.02 eV, Table 1) is smaller than observed for its non-helical isomer DIPh.12 Compound 2 features a weak absorption extending beyond 1500 nm, which implies a highly radicaloid nature of the π system, whereas the largest energy gap is observed for 3. Electrochemical energy gaps obtained from voltammetric measurements are larger by ca. 0.3–0.4 V but follow the trend seen in optical data. All helicenes displayed one reversible oxidation occurring at relatively low potentials (0.17–0.21 V vs Fc/Fc+), suggesting that their HOMO levels are destabilized and have similar energies. Interestingly, 1 is more easily oxidized than DIPh (0.26 V), implying a relatively greater stabilization of its monocation. Each helicene features two electrochemically reversible one-electron reductions. The reduction potentials decrease in the order 2>1>3, with a particularly high Ered,1=−0.97 V found for 2.

Redox behavior and absorption spectra of 1–3. Left: cyclic and differential pulse voltammograms measured in DCM solution with TBAPF6 electrolyte. Centre: UV/Vis-NIR spectra of 1–3 (1 mM in DCM, 1 mm path length) titrated with NOSbF6. Right: UV/Vis-NIR spectra of 1–3 (1 mM in THF, 1 mm path length) titrated with sodium anthracenide. Intermediate stages of titration from neutral compounds to radical cations and radical anions are shown in gray.
System |
EgE a (eV) |
EgO b (eV) |
ΔEST (kcal/mol) c |
y0 d |
||
|---|---|---|---|---|---|---|
SQUID e |
DFT f |
CAS g |
DFT f |
|||
1 |
1.30 |
1.02 |
−3.5 |
−1.3 |
0.73 |
0.63 |
2 |
1.15 |
0.79 |
≈0.0 |
1.0 |
0.92 |
0.89 |
3 |
1.70 |
1.25 |
<−7 |
−11.7 |
0.08 |
0.00 |
- a Electrochemical energy gap. b Optical energy gap. c Singlet–triplet gap ΔEST=ES-ET. d Diradicaloid index of the open-shell singlet state, defined as equal to the LUNO occupancy.56 e From magnetic susceptibility measurements in the solid state. f Calculated (UB3LYP/6-11++g(d,p)//UB3LYP/6-31g(d,p), gas phase). g CASSCF(2,2)/6-31g(d,p).
Chemical oxidation with [NO][SbF6] confirmed that the radical cations 1⋅+, 2⋅+, and 3⋅+ can be generated in stoichiometric amounts and are stable in solution. Each cation features an intense NIR band, which is red-shifted relative to the absorption onset of the corresponding neutral state. The 1⋅+ radical cation is notable for its uniquely low-energy absorption (λmax≈2600 nm, Figures 5 and S5), which is bathochromically shifted by nearly 600 nm relative to the corresponding band of [DIPh]⋅+. Interestingly, the radical monoanion 1⋅−, obtained by reduction of 1 with sodium anthracenide in THF, displays a similar, but even more intense NIR band (λmax≈2500 nm, Figure S6). A comparable, yet much weaker absorption was observed in the spectrum of 2⋅−, accompanied by a more intense NIR feature at 1201 nm. In contrast, the radical anion 3⋅− showed no observable bands above 1500 nm. Further chemical reduction of 1⋅− produced the dianion 12− with no NIR absorptions. A similarly large energy gap was observed for the dianion 22−, whereas the dianion 32− produced a more extended absorption up to ca. 800 nm. The distinct optical characteristics of the charged states of 3 may be attributed to its unique ring system, wherein a phenalenyl subunit replaces one of the indenyl fragments.
Further experiments showed that the paramagnetism of the three helicenes increases in the order 3<1<2. Compound 3 was the only one to yield a sharp 1H NMR spectrum at 300 K (Figure S3). In contrast, the 1H NMR spectrum of 1 in DCM-d2 was significantly broadened at 300 K, and the sample had to be cooled to 180 K to produce sufficiently sharp signals (Figures S1, S2), implying the contribution of a thermally accessible triplet state at higher temperatures. In the case of 2, the aromatic region was unobservable at 300 K in CDCl3, suggesting an even higher population of the triplet state. The solid-state room-temperature ESR spectrum of 1 showed a signal with resolved triplet components, which could be modeled in the rhombic symmetry to yield zero-field splitting (zfs) parameters D=0.0115 cm−1 (123 G) and E=0.0022 cm−1 (24 G, Figure 6A). The D value corresponds to an effective dipole−dipole interaction distance of 6.09 Å,57 shorter than the geometrical separation between the formal radical sites in 1 (7.1–7.2 Å, XRD data). This discrepancy may result from delocalization of spin density into the terminal benzene rings of the helicene, with the minimum C⋅⋅⋅C separation of ca. 3.3 Å. Indeed, the relatively strong zfs is apparently a unique feature of the compact helicene structure of 1, as it was not observable in its non-helical isomers or more π-extended analogs.12, 13, 28 DFT calculations yielded D=−0.0097 cm−1 and E/D=0.11, in good agreement with the experimental values.
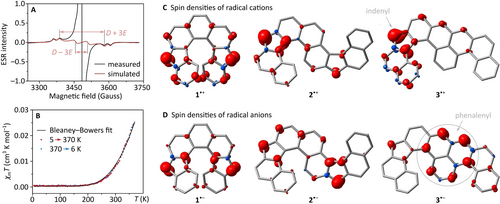
(A) Electron spin resonance (ESR) spectrum of 1 (powder sample, room temperature). The large central peak corresponds to trace monoradical impurities and is absent in the simulated spectrum. (B) Temperature dependence of magnetic susceptibility of 1 determined using superconducting quantum interference device (SQUID) magnetometry. (C−D) Spin densities of radical cations and anions of 1–3 (UB3LYP/6-31G(d,p), 0.005 a.u. isovalue).
Solid samples of 1 displayed a positive temperature dependence of the ESR signal intensity, which could be fitted with the Bleaney–Bowers model to produce a ΔEST value of −3.8 kcal/mol (Figure S12). A closely matching ΔEST value (−3.5 kcal/mol) was obtained for 1 from the temperature dependence of molar magnetic susceptibility, expressed as χmT(T), determined in a SQUID measurement (Table 1, Figure 6B). In comparison, χmT of the double helicene 2 was much higher at a value of 0.75 cm3K/mol and nearly independent of temperature. It only decreased below 15 K, likely indicating a weak antiferromagnetic coupling (Figure S19). This behavior is consistent with near-degenerate singlet and triplet states, as opposed to a definitive triplet ground state, for which χmT close to 1 cm3K/mol would be expected. While solid 2 yielded an ESR line with no observable zfs features, weak triplet components were observed in frozen solution. These features could be modeled in the axial symmetry with D=0.0056 cm−1 (60 G). The weaker zfs in comparison to 1 corresponds to the larger geometrical separation of spin centers in 2 (8.83 Å, XRD data). Compound 3 did not display any increase of χMT up to 500 K. The upper bound of ΔEST deduced from this measurement (−7 kcal/mol, Table 1) agrees with the computational estimate. The residual ESR signal and temperature-independent χMT of 0.01 cm3K/mol observed for 3 can be ascribed to a paramagnetic impurity at a level of ca. 2–3 %.
Diradicaloid indices y0 obtained for the singlet states using complete active space (CAS) and DFT calculations (Table 1) indicate a predominantly closed-shell configuration for 13 (y0 <0.1) and a highly open-shell character of 12 (y0≥0.9). The singlet 11 is an intermediate case, with y0 close to the value reported for the isomeric DIPh (0.69).12 These values can be rationalized using Clar-sextet counts (NCS) of the closed- and open-shell configurations of helicenes (1–3 vs. 1′–3′, respectively, Figure 1). The low open-shell character of 3 is explained by the modest gain of one Clar sextet in the open-shell form 3′. 1 and 2 gain two Clar sextets each upon electron unpairing; the larger size of 2 (4 Clar sextets per 9 fused rings) may account for it's particularly high y0 index. The calculated singlet–triplet gap (ΔEST) increases with the decreasing y0; in particular, a value of 1.0 kcal/mol is predicted for 2. While the accuracy of DFT estimates is limited, the above value indicates the feasibility of the triplet ground state, or a near degeneracy of the two multiplicities, in line with the SQUID results. The value of ΔEST calculated for 1 is consistent with the ground state singlet and a thermally accessible triplet, whereas the relatively large negative gap obtained for 3 implies that the triplet should be insignificantly populated at accessible temperatures.
Spin densities obtained from DFT calculations for broken-symmetry singlets and triplets of 1–3 (Figure S8) show that the unpaired electrons are delocalized over the entire π-system, with the largest amplitudes at the formal radical sites (cf. 1′–3′, Figure 1). The spin appears to be more localized in the charged radicals 1⋅+ and 1⋅−, in which the spin density is symmetrically distributed over the two arms of the helicene (Figure 6C–6D). Interestingly, in the corresponding radicals 2⋅+ and 2⋅−, the spin is partitioned unequally: larger spin contributions are found in the nor[6]helicene unit of the radical cation and in the nor[5]helicene section of the radical anion, respectively. An even more pronounced difference is found in the radical ions of 3, showing a spin distribution characteristic of phenalenyl radical in 3⋅−, and indenyl radical in 3⋅+. Electrostatic potential maps (Figure S9) demonstrate complementary charge distributions, i.e., with the positive and negative charge concentrated, respectively, in the phenalenyl part of 3⋅+ and the indenyl part of 3⋅−.
Nucleus-independent chemical shift (NICS) scans, performed for substituent-free models 1H–3H (Figure 1, R=H), were used to assess the aromaticity of the three helicenes in their singlet and triplet states (Figure 7). The singlet of nor[7]helicene, 11H, displayed strong paratropicity (i.e., large positive NICS values) in the five-membered rings B and F, as typically observed in diindenoarenes. The terminal benzene rings A and G were quite strongly diatropic, whereas the diatropicity of the inner phenanthrene fragment was diminished (rings C−E), indicating considerable quinoidal contributions to the electronic structure. In the triplet state 31H, the aromaticity of the inner section of the π system noticeably increases, as evidenced by the lowering of NICS values in rings B−F. NICS scans obtained for 2H are remarkable for their apparent mirror symmetry, which implies that the aromaticity of outer cyclopenta[a]naphthalene fragments (rings A−C and G−I, respectively) is similar in spite of the different connectivities of these two units with the central phenanthrene. The phenanthrene fragment (rings D−F) appears less diatropic in 12H than in 11H, suggesting that in the former system the benzenoid aromaticity is disrupted to a greater extent by open-shell contributions. This observation is surprising given the apparent weak interaction between spins in 2. NICS values decrease in the triplet state 32H, similarly as in the nor[7]helicene triplet, indicating partial recovery of global aromaticity caused by electron unpairing.
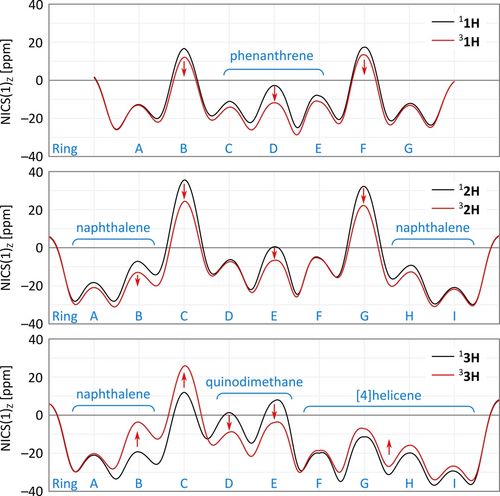
NICS(1)Z scans performed for singlet and triplet states of 1H–3H. The horizontal coordinate corresponds to a zigzag pathway linking centers of consecutive 5- and 6- membered rings, displaced 1 Å above the surface of the molecule. The NICS value plotted is the negative magnetic shielding in the direction of displacement. Ring labels are defined in Figure 2; for scan paths, see Figure S11.
The NICS scan of 3H differs dramatically from the profiles obtained for the diindeno systems 1H and 2H, reflecting the influence of the ring fusion pattern. In the singlet state 13H, the naphthalene and [4]helicene sections (A−B and F−I, respectively) are distinctly diatropic, in line with their benzenoid aromaticity. The remaining section of the π system (rings C−E) shows positive NICS values above ring centers, which agree with the partial quinodimethane character of this section inferred from structural data. The triplet state 33H features the expected increase of aromaticity in the quinodimethane section, however, the outer naphthalene and [4]helicene units actually become less aromatic. This finding rationalizes the large negative ΔEST gap predicted for 3 as well as its experimentally observed diamagnetism.
Conclusion
The approach to open-shell helicenes described in this work combines an annulative synthesis of phenanthrenes with Friedel–Crafts cyclization, to produce curved polycyclic frameworks. The steric congestion induced in the first of these two steps results in divergent outcomes, yielding [n]helicene substructures with n ranging from 4 to 7. The resulting helicene products reveal the intricacies of underlying mechanistic pathways, while also providing access to structurally diverse diradicaloids with varied electronic characteristics. Differences in the topology of the π system determine the strength of spin–spin interactions in the neutral diradicaloid states, while also affecting spin and charge delocalization in the radical ions. We believe that, by further optimization of reaction conditions or judicious selection of target structures, the present method can be elaborated to afford higher open-shell helicenes containing even stronger through-space interactions between radical centers as well as multi-helicene systems with non-trivial π conjugation. Because of the unique steric features of these systems, they will be not only persistently chiral but may also exhibit enhanced chemical stability, which is an essential requirement in practical applications of radicaloid materials.
Acknowledgments
Financial support was kindly provided the National Science Center of Poland (UMO-2019/35/B/ST4/00401 to M.S.). Theoretical calculations were performed using the resources provided by the Wrocław Center for Networking and Supercomputing. We thank Dr. Miłosz Siczek for his assistance with X-ray diffraction measurements.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.