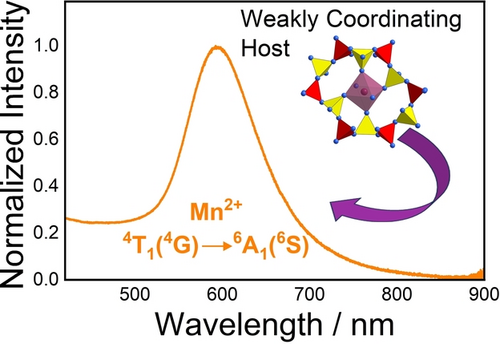
Photoluminescence of Mn2+ in the Borosulfate Zn[B2(SO4)4] : Mn2+—A Tool to Detect Weak Coordination Behavior of Ligands
Graphical Abstract
The luminescence of Mn2+ is strongly affected by the ligand field. Usually only the metal-ligand distance is considered a tool to tune the ligand field strength in phosphors. Using Zn[B2(SO4)4] as a host, we show that electronic effects are an important alternative and lead to unusual orange luminescence of Mn2+.
Abstract
The impact of the surrounding ligand field is successfully exploited in the case of Eu2+ to tune the emission characteristics of inorganic photoactive materials with potential application in, e.g., phosphor-converted white light-emitting diodes (pc-wLEDs). However, the photoluminescence of Mn2+ related to intraconfigurational 3d5–3d5 transitions is also strongly dependent on local ligand field effects and has been underestimated in this regard so far. In this work, we want to revive the idea how to electronically tune the emission color of a transition metal ion in inorganic hosts by unusual electronic effects in the metal-ligand bond. The concept is explicitly demonstrated for the weakly coordinating layer-like borosulfate ligand in the Mn2+-containing solid solutions Zn1-xMnx[B2(SO4)4] (x = 0, 0.03, 0.04, 0.05, 0.10). Zn[B2(SO4)4]:Mn2+ shows orange narrow-band luminescence at 590 nm, which is an unusually short wavelength for octahedrally coordinated Mn2+ and indicates an uncommonly weak ligand field. On the other hand, the analysis of the interelectronic Racah repulsion parameters reveals ionic Mn−O bonds with values close to the Racah parameters of the free Mn2+ ion. Overall, this strategy demonstrates that electronic control of the metal-ligand bond can be a tool to make Mn2+ a potent alternative emitter to Eu2+ for inorganic phosphors.
Introduction
In recent years, an overwhelming amount of new phosphor materials has emerged to meet versatile industrial and scientific requests. Most of these phosphors are Ce3+- or Eu2+-doped oxide or nitride-based ceramics, which show luminescence based on electric dipole-allowed 4fN−15d1→4fN transitions (N=1 for Ce3+, N=7 for Eu2+).1-6 In order to meet the numerous requirements for applications in light-emitting devices and displays, the employed host compounds for these activator ions usually combine structural rigidity and high coordination number with high chemical and thermal resistance to induce desirable luminescence features like narrow-band emission, high quenching temperatures and high quantum efficiency. Famous commercialized examples are Y3Al5O12 : Ce3+ (YAG : Ce3+),7 Sr2Si5N8 : Eu2+,8 Sr[LiAl3N4] : Eu2+ (SLA : Eu2+)9 and Sr[Li2Al2O2N2] : Eu2+ (SALON : Eu2+).10 Besides those, Eu2+ activated CaAlSiN3,11 alkali lithooxidosilicates,12-17 (oxo−)nitridoberyllates18-21 and oxynitrides22, 23 have attracted attention due to their favorable luminescence characteristics. Despite the promising photoluminescence properties of Eu2+ for next-generation phosphor-converted white light-emitting diodes (pc-wLEDs), the more abundant transition metal ion Mn2+ could be a potential alternative in this field. Since the photoluminescence of Mn2+ relies on electronic transitions within the spatially less extended 3d subshell compared to the diffuse 5d orbitals of Eu2+, narrower and more color-pure emission bands can generally be expected from Mn2+ if appropriately optimized. Moreover, given the spatially extended nature of the 3d orbitals, the emission color of Mn2+ can be as easily controlled by the coordination geometry and type of surrounding ligands as for Eu2+. Tetrahedrally coordinated Mn2+ usually exhibits narrow-band green luminescence, e.g. in MgAl2O4 : Mn2+ (λem=525 nm),24 Zn2SiO4 : Mn2+ (λem=525 nm),25, 26 ZnGa2O4 : Mn2+ (λem=500 nm),27 BaZnAl10O17 : Mn2+ (λem=516 nm),28 LaMgAl11O19 : Mn2+ (λem=517 nm),29 Sr2MgAl22O36 : Mn2+ (λem=518 nm),30 Zn4B6O13 : Mn2+ (λem=540 nm).31 In contrast, octahedrally coordinated Mn2+ typically shows red emission, e.g. MgSiO3 : Mn2+ (λem=661 nm),32 Mg4Ta2O9 : Mn2+ (λem=659 nm),26 Ba2M(BO3)2 : Mn2+ (M=Mg, Ca; λem=615 nm, 630 nm),33, 34 NaZn(PO3)3 : Mn2+ (λem=613 nm, 643 nm).35 This is related to a stronger ligand field splitting in an octahedral field and accompanied by larger emission bandwidths as well as generally lower quenching temperatures (T50).36 The marked difference in the emission properties for tetrahedrally and octahedrally coordinated Mn2+ is understandable from the d5 Tanabe-Sugano diagram valid for both cases (see Figure 1). The lowest energetic excited ligand field state 4T1(g)(4G) of a d5 ion decreases in energy relative to the ground state 6A1(g)(6S) with increasing ligand field strength. The spin-forbidden nature of the optical transitions of Mn2+ in a 3d5 high-spin configuration leads to a generally long emission decay time, which may pose problems to high-power pc-wLEDs based on saturation effects (cf. the related emitter Mn4+).37 The decay time can be, however, shortened upon incorporation into hosts with heavier atoms due to enhanced spin-orbit coupling or upon exploitation of magnetic exchange interaction in Mn−Mn pairs.39 Often is only an ionic picture of the crystal field interaction considered and its strength is generally enhanced by decreasing Mn−ligand distances as well as a transition from tetrahedral to octahedral coordination geometry. However, the impact of covalency and a decrease in the interelectronic repulsion parametrized by the Racah parameters B and C is as important and can also be used to tune the ligand field strength. Nitrides with tetrahedrally coordinated doping sites for Mn2+ serve as an illustrative example here. AlN : Mn2+ (λem=600 nm),40 ZnGeN2 : Mn2+ (λem=610 nm)41 and MgSiN2 : Mn2+ (λem=630 nm)42 are red-emitting phosphors despite tetrahedral coordination of the doped Mn2+ ions, which is a consequence of the strong σ-donating nature of the N3− ligands and the resulting strong degree of covalency of the Mn−N bonds. In contrast, one would expect a blue-shifted luminescence of octahedrally coordinated Mn2+ ions if the surrounding ligands were weakly coordinating with a high degree of ionicity in the Mn-ligand bond. This strategy has so far only been scarcely regarded. An alternative approach to obtain narrow-band emitting phosphors based on Mn2+ with other emission colors than green is thus to embed them in an octahedral ligand field with only weakly coordinating ligands. Similar effects are already known for Eu2+ in selected borates e.g. SrB4O743, 44 and aluminates45 or very ionic fluorides such as AMgF3 (A=K, Rb),46, 47 for which in the latter example, even the observation of 4f7-4f7 narrow-line luminescence of Eu2+ was possible.
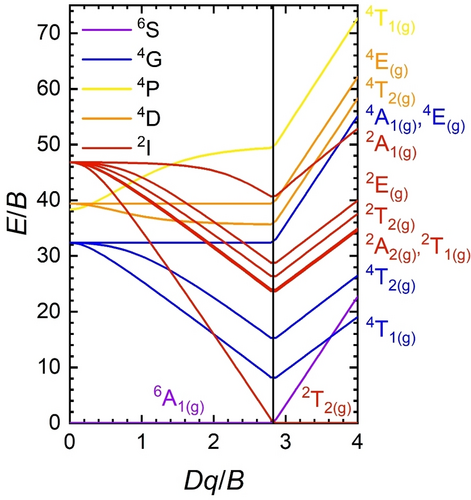
Tanabe-Sugano diagram (C/B=4.5) for octahedrally coordinated d5 ions.38 B and C denote the interelectronic repulsion Racah parameters.
Years ago, borosulfates as a new class of silicate-analogue compounds started to gain increasing interest and in recent years some borosulfate anions were characterized as weakly coordinating, making them promising candidates for our investigations.48-50 Typically, the anionic substructures of borosulfates consist of vertex-connected (BO4)- and (SO4)tetrahedra formally substituting the (SiO4)tetrahedra in silicates.51 The anionic substructures comprise e.g. oligomeric anions (like nesosilicates),52, 53 infinite chains (like inosilicates),53, 54 layers (like phyllosilicates)50, 55-57 or 3D network structures (like tectosilicates).58, 59 The weak coordination behavior was also demonstrated by the stabilization of (I4)2+-cations.60 The latter can also be obtained in presence of a sufficiently powerful oxidizer and weakly coordinating anions such as [AsF6]− and [SbF6]−.61 The special reaction conditions during the synthesis as well as the weak coordination behavior of the borosulfate anions are most likely also the reason for the stabilization of the cluster-like heteropolycations in [Au3Cl4][B(S2O7)2] and the chain-like heteropolycations in [Au2Cl4][B(S2O7)2](SO3).62 Spectroscopic evidence for the weak coordination behavior of borosulfates has so far been mainly related to the luminescence of lanthanoid ions such as Ce3+ or the UV/Vis absorption of transition metal ions such as Co2+ or Ni2+.48, 50, 63
Based on these promising findings, it was our goal to demonstrate that weakly coordinating ligands can be beneficially employed to tune the photoluminescence properties of Mn2+ for alternative lighting sources in a desirable manner and that the covalency of the Mn-ligand bond plays an equally important role in controlling of the ligand field strength.
Results and Discussion
Zn[B2(SO4)4] crystallizes in the space group P21/n (no. 14), with the lattice parameters a=7.8338(4), b=8.0967(4), c=9.0399(4) Å and β=111.26(1)°.57 It features heteropolyanionic layers of alternating (BO4)- and (SO4)-tetrahedra sharing a common vertex. Each (BO4)-tetrahedron is connected to four (SO4)-tetrahedra, which themselves are connected only to two (BO4)-tetrahedra, thus exhibiting two terminal oxygen atoms. The layers consist of vierer- and zwölfer-rings of alternating tetrahedra, a structural motif which can be found in a lot of conventional phyllosilicate-analogue borosulfates. Inside the twelve-membered rings the charge compensating Zn2+ cations reside, octahedrally coordinated by six oxygen atoms; four belonging to the surrounding ring and two denote to the adjacent polyanionic layers. All oxygen atoms coordinating the Zn2+ cation are terminal oxygen atoms of the (SO4)-tetrahedra. Figure 2 depicts the structure and coordination of the cation in Zn[B2(SO4)4] : Mn2+, both within the layer (Figure 2b) and by two oxygen atoms of adjacent layers, which are situated in a trans arrangement within the coordination octahedron (Figure 2a).
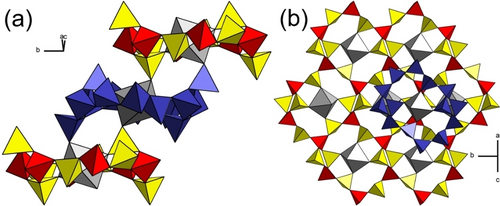
Structural features of Zn[B2(SO4)4]. (a) View parallel to the layers. (b) View orthogonal to the layers. Color code: yellow—(SO4)-tetrahedra, red—(BO4)-tetrahedra, gray—((Zn/Mn)O6)-octahedra. One layer is colored blue as a guide for the eye.
Figure 3 shows a representative Rietveld refinement of the solid solution series Zn[B2(SO4)4]: x%Mn2+ (x=0.5, 1, 2, 3, 4, 5, 10). The percentage of dopant was fixed during the refinement. Further details of the synthesis and the fitting quality parameters of the Rietveld refinement are given in the Supporting Information.
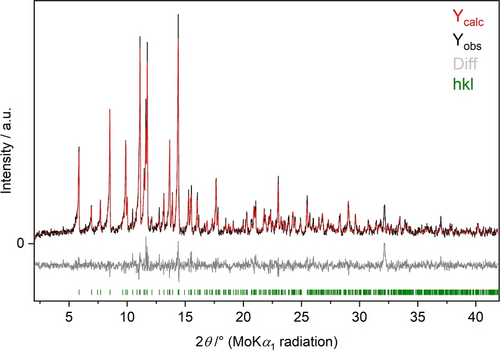
Rietveld-refined XRPD pattern of Zn[B2(SO4)4]: 10 % Mn2+ (MoKα1 radiation). For details on the quality parameters of the refinement, see the Supporting Information.
Luminescence Spectroscopy
All of the Mn2+-activated Zn[B2(SO4)4] samples, enclosed in quartz ampoules, with high optical quality, showed luminescence upon excitation with UV light. Figure 4a depicts the photoluminescence emission (PL) and excitation (PLE) spectra of Zn[B2(SO4)4]: 10 % Mn2+ at room temperature. An intense, broad orange emission is observed under excitation with 290 nm. The emission band is located at 595 nm with a full width at half maximum (FWHM) of 2960 cm−1 (≈103 nm in the wavelength domain) and the emission is assigned to the 4T1g(4G)→6A1g(6S) transition according to the Tanabe-Sugano diagram. The PL and PLE spectra were also recorded at 77K but no additional information could be gained due to limited emission intensity of the compounds in evacuated quartz ampoules. Figure 4b depicts the luminescence decay trace of the 4T1g(4G)→6A1g(6S) transition of Mn2+. It shows multi-exponential decay with an average decay time of 21 ms. The luminescence characteristics agree with the structural feature of octahedrally coordinated Mn2+. The decay time of 21 ms is longer than the commonly reported decay times for tetrahedrally coordinated Mn2+ (e.g. MgAl2O4: 3 % Mn2+: τ=6.5 ms at room temperature;24, 39 [Bz(Me)3N]2[MnCl4]: τ=3.8 ms at room temperature ([Bz(Me)3N]+: Benzyl-trimethylammonium);64 Zn2SiO4: 0.05 % Mn2+: τ=13 ms at room temperature).65 Even if the regarded high doping fraction of 10 % Mn2+ is taken into account, the extracted decay time of 19 ms at room temperature already indicates that violation of the spin selection rule cannot be the sole reason for the slow decay, but the transition must be also parity-forbidden. This goes in line with examples of octahedrally coordinated Mn2+ ions in inorganic host compounds such as KMgF3: 0.5 % Mn2+ (τ=100 ms at room temperature) or selected perovskite-derived chlorides AMCl3 : Mn2+ (A=K, Rb, Cs; M=Mg, Ca, Sr) (τ≈20 ms at room temperature) with slightly distorted octahedral doping sites for the Mn2+ ions.66, 67 In contrast, the FWHM of the Mn2+-related emission band in Zn[B2(SO4)4] : Mn2+ is comparably small and in a similar order of magnitude compared to reported values for the FWHMs of emission bands for tetrahedrally coordinated Mn2+ (in halides: 1400 cm−1–2000 cm−1 at room temperature).64 Moreover, the emission wavelength is comparably short for octahedrally coordinated Mn2+, which usually extends beyond 600 nm (e.g. MgSiO3 : Mn2+: λem=661 nm,32 CsMnBr3: λem=680 nm,68 Ba2Ca(BO3)2 : Mn2+: λem=630 nm33) and only becomes orange in weak octahedral ligand fields (AMCl3 : Mn2+: λem≈560 nm,67 CsPbCl3 : Mn2+: λem=586 nm,69 MgF2 : Mn2+: λem=586 nm70). Altogether, these properties demonstrate that the Mn2+ ions are octahedrally coordinated in Zn[B2(SO4)4] : Mn2+ but with only weakly coordinating ligands. This is in agreement with earlier findings by Höppe et al. on lanthanoid-activated borosulfates.48, 71 The weak coordination behavior is understandable given the presence of the strongly electron-withdrawing SVI atoms that compete with Mn2+ about the electron density at the 2p lone pair of a coordinating O atom. In agreement with this interpretation, the luminescence decay time barely changes between 77 K and room temperature (see Figure 4b). The photoluminescence excitation spectra (PLE) of Zn[B2(SO4)4]: 10 % Mn2+ shows characteristic transitions of Mn2+ that can be assigned according to the d5 Tanabe-Sugano diagram (see Figure 1). All these transitions are spin- and parity-forbidden in an octahedral ligand field. Furthermore, a weak bluish emission is also observed, which is attributed to host-related luminescence but could not specified further. Even in nominally undoped Zn[B2(SO4)4], a broad-banded emission peaking at around 550 nm can be observed at room temperature (see Supporting Information). The intensity of this host emission is small upon excitation with 290 nm and is only dominant in samples with low Mn2+ content (3 mol % or below, see Supporting Information). Figure 5 shows the PL properties of Mn2+-activated Zn[B2(SO4)4] with varying Mn2+-concentrations. The 4T1g(4G)→6A1g(6S)-based emission band of the Mn2+ ions is slightly red-shifted for the higher doped samples, which is a consequence of exchange interaction between different Mn2+ ions.38 However, given a relatively long closest Zn−Zn distance of 6.2707(2) Å in Zn[B2(SO4)4],56 the effect of mutual interactions between the incorporated Mn2+ ions is limited. Exchange interaction between the Mn2+ ions is usually accompanied by an increasing FWHM and a gradual decrease of the decay time together with increasingly multi-exponential decay behavior.38 Both the dependence of the emission FWHM and average luminescence decay times show that exchange coupling and concentration quenching is weak up to the regarded doping concentrations of 10 mol %.
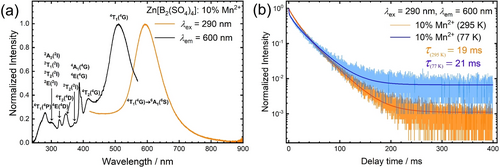
Photoluminescence properties of Mn2+ in Zn[B2(SO4)4]. (a) PL and PLE spectra of Zn[B2(SO4)4]: 10 % Mn2+ at room temperature. Assignment of states according to the Tanabe-Sugano diagram. The 10 % Mn2+-activated compound was chosen as representative sample due to its favorable ratio of Mn2+-based to host-based luminescence. Note that the parity of the states was omitted for representational reasons. (b) PL decay curves of Zn[B2(SO4)4]: 10 % Mn2+ at 295 K (orange) and 77 K (blue). Excitation and emission wavelength are indicated in the graph. Decay times are averaged values extracted from multiexponential fit functions.
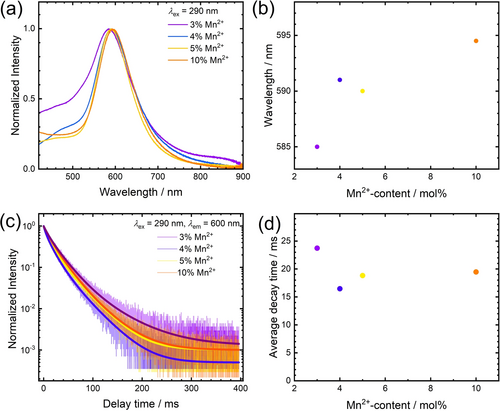
Photoluminescence of different doping concentrations of Zn[B2(SO4)4] : Mn2+. a) PL emission spectra at room temperature. The excitation wavelength is indicated in the graph. b) Plot of the wavelength at maximum emission intensity versus Mn2+ concentration. c) Time-resolved luminescence of Mn2+ at room temperature. Excitation and emission wavelengths are indicated in the graph. d) Plot of the average decay time at room temperature versus Mn2+ content.
It should be noted that also an effect due to a change in the average Mn−O bond length is imaginable, which could affect the ligand field splitting based on its R−5 dependence for d-elements.72, 73 Since Mn2+ in its 3d5 high-spin configuration has, however, a larger ionic radius than Zn2+ with a 3d10 configuration, one would expect an overall decreasing ligand field strength giving rise to an observable blue-shift of the emission based on the d5 Tanabe-Sugano diagram (see Figure 1). Since this is not experimentally observed, we conclude that this contribution is not the dominant one.
Racah parameters and ligand field analysis
From the energies of the electronic transitions, it is possible to estimate the Racah parameters B and C and the ligand field splitting 10Dq as B=729 cm−1, C=3660 cm−1 and 10Dq=9430 cm−1 respectively (a detailed description of the calculations is given in the Supporting Information).74 The same approach was applied for some other selected compounds with Mn2+ in halides with octahedrally coordinated sites with the results being compared in Table 1. It should be noted that this semi-empirical approach only offers estimate values for interelectronic repulsion and ligand field parameters of Mn2+, which allows, however, a quantitative comparison between the regarded compounds. More elaborate ab initio methods would be required to gain additional accuracy in especially the Racah parameters, which are, however not trivial for open-shell systems and usually requires wavefunction-based approaches.75-78 Nonetheless, clear trends are already visible at this stage that also agree with the experimentally found emission and lowest excitation energies. The Racah parameter B for Zn[B2(SO4)4] : Mn2+ is exceptionally high, even in comparison with highly ionic fluorides. Consequently, the Mn−ligand bond may be regarded as strongly ionic. The ligand field splitting caused by the borosulfate anion (10Dq=9430 cm−1) is in between the values calculated for chlorides (10Dq≈8500 cm−1) and fluorides (10Dq≈10000–11000 cm−1). Together with the strong ionic degree of the Mn−ligand bond, the borosulfate anion indeed shows characteristic weak coordination behavior that is directly reflected in the photoluminescence properties of the incorporated Mn2+ cations.
|
Zn[B2(SO4)4] : Mn2+ |
CsCaCl3 : Mn2+[79] |
KMgCl3 : Mn2+[79,80] |
CsCaF3 : Mn2+[81] |
KZnF3 : Mn2+[82] |
|---|---|---|---|---|---|
B/cm−1[a] |
729 |
631 |
619 |
702 |
696 |
C/cm−1[a] |
3663 |
3579 |
3537 |
3666 |
3651 |
10Dq/cm−1[a] |
9427 |
8583 |
8629 |
9844 |
11285 |
Dq/B |
1.29 |
1.36 |
1.39 |
1.40 |
1.62 |
C/B |
5.0 |
5.7 |
5.7 |
5.2 |
5.2 |
Eex/cm−1 |
19430 |
20095 |
19440 |
19880 |
18530 |
Eem/cm−1 |
16680 |
18190 |
17530 |
18350 |
17100 |
- [a] Values were derived from excitation spectra without Racah-Trees or seniority correction and may therefore differ from values in the respective reference.
Conclusion
Mn2+ is a so-far underestimated potential emitter for inorganic phosphors given its less spatially extended 3d orbitals compared to the 5d orbitals exploited in the celebrated case of Eu2+. In most studies, the emission color is varied by changes in either the metal−ligand distances or the coordination geometry. Tetrahedrally coordinated Mn2+ typically emits in the green, octahedrally coordinated Mn2+ typically in the red range. An alternative concept to tune the emission color of transition metal ions can be electronic control of the nature of the metal-ligand bond. This concept was explicitly demonstrated in the phyllosilicate-analogous borosulfates Zn1-xMnx[B2(SO4)4] (x=0, 0.03, 0.04, 0.05, 0.10) with weakly-coordinating borosulfate ligands. Zn[B2(SO4)4]: x% Mn2+ (x=3, 4, 5, 10) shows an orange 4T1g(4G)→6A1g(6S) emission at≈590 nm, which is unusually high in energy for Mn2+ in an octahedral coordination of an oxidic compound and indeed implies a weak coordination behavior of the ligands according to the d5 Tanabe -Sugano diagram. By comparison with literature-known examples of Mn2+-activated symmetric fluorides and chlorides with octahedrally coordinated doping sites for this activator, we were able to place the borosulfate ligands into the commonly known spectrochemical series of ligands just between Cl− and F−. These studies give an insight into more sophisticated possibilities to tailor the emission color of Mn2+ in inorganic phosphors by electronic means with chemically more uncommon ligands.
Acknowledgments
J. B. gratefully acknowledged support by the Max-Buchner-Forschungsstiftung (No. 3817). M. S. and J. B. are grateful for a materials cost allowance by the Fonds der Chemischen Industrie e.V. and M. S. acknowledges funding by the “Junges Kolleg” of the North-Rhine Westphalian Academy of Sciences and Arts. L.C.P. appreciates the PhD scholarship from the Universität Innsbruck. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.