Solvent-Free Chemical Recycling of Polymethacrylates made by ATRP and RAFT polymerization: High-Yielding Depolymerization at Low Temperatures
Graphical Abstract
A low temperature and solvent-free method to depolymerize polymers made by ATRP and RAFT is reported. By performing an efficient end-group modification, the modified polymers were subsequently successfully depolymerized, resulting in up to 90 % monomer regeneration at temperatures as low as 220 °C. Importantly, the process was scalable and the monomer produced had high purity and could subsequently be used to make a new batch of polymer.
Abstract
Although controlled radical polymerization is an excellent tool to make precision polymeric materials, reversal of the process to retrieve the starting monomer is far less explored despite the significance of chemical recycling. Here, we investigate the bulk depolymerization of RAFT and ATRP-synthesized polymers under identical conditions. RAFT-synthesized polymers undergo a relatively low-temperature solvent-free depolymerization back to monomer thanks to the partial in situ transformation of the RAFT end-group to macromonomer. Instead, ATRP-synthesized polymers can only depolymerize at significantly higher temperatures (>350 °C) through random backbone scission. To aid a more complete depolymerization at even lower temperatures, we performed a facile and quantitative end-group modification strategy in which both ATRP and RAFT end-groups were successfully converted to macromonomers. The macromonomers triggered a lower temperature bulk depolymerization with an onset at 150 °C yielding up to 90 % of monomer regeneration. The versatility of the methodology was demonstrated by a scalable depolymerization (≈10 g of starting polymer) retrieving 84 % of the starting monomer intact which could be subsequently used for further polymerization. This work presents a new low-energy approach for depolymerizing controlled radical polymers and creates many future opportunities as high-yielding, solvent-free and scalable depolymerization methods are sought.
Introduction
The development of controlled radical polymerization (CRP) methods, and in particular atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer (RAFT) polymerization, has revolutionized polymer science, as well-defined vinyl polymers can be prepared under mild conditions with unprecedented control over molecular weight, molecular weight distribution, architecture and composition.1-8 The unparalleled success of these techniques lies in their ability to preserve the end-group fidelity of the vast majority of polymer chains, which not only helps to maintain control over the polymerization, but also allows a plethora of complex materials to be prepared.9-13 However, while polymer chemists have focused almost exclusively on developing the synthesis, very limited reports exist on reversing the process, i.e. achieving depolymerization by chemically breaking down polymers back into their starting monomers, which can then be repolymerized into virgin-like materials.14-19 Although excellent degradation strategies have been widely developed, these approaches focus on breaking down the polymer into small molecules rather than retrieving the starting monomer.20-25 Instead, chemical recycling is essential if we are going to make polymers more sustainable, reduce waste and prevent their continued accumulation in the environment.26-29
Achieving depolymerization of vinyl polymers is particularly challenging, as the carbon-carbon bonds that form the polymer backbone have extremely high thermal stability.30 Nevertheless, remarkable achievements have emerged recently with the development of solution-based depolymerization methods for polymers made by CRP.18, 31 Seminal work from the group of Ouchi illustrated that in the presence of a ruthenium catalyst, it was possible to depolymerize poly(methyl methacrylate) (PMMA) obtained from ATRP.32 Matyjaszewski and co-workers very impressively illustrated that both copper and iron catalysts could be exploited to depolymerize a wide-range of methacrylate polymers, obtaining around 70 % of the original monomer at temperatures as low as 170 °C.33-35 In addition, polymers obtained from RAFT polymerization have been depolymerized by our laboratory and those of Gramlich and Sumerlin.36-40 Notably, these methods have been developed to recover as much as 90 % of the original monomer at temperatures as low as 120 °C, or even at 100 °C in the presence of UV or blue light irradiation.36-40
Despite the aforementioned outstanding results, the vast majority of current approaches operate best in specific solvents, such as dioxane, while significantly lower depolymerization conversions are reported in alternate media, thus limiting the scope of depolymerizable materials.37-40 In addition, very high polymer dilutions (i.e. 0.1 mM polymer concentration or 5 mM repeat unit concentration) are typically essential to favour a successful depolymerization.37-40 When higher polymer concentrations have been employed (i.e. 12–25 mM polymer concentration or 100–750 mM repeat unit concentration), higher temperatures were required (e.g. 170 °C) in conjunction with significant amounts of copper or iron ATRP catalysts.33-35 As such, a scalable and both catalyst and solvent-free (i.e. bulk) approach to depolymerize ATRP and RAFT materials would be highly beneficial and could potentially attract considerable attention from both academia and industry. However, to date current reports require high temperatures to facilitate random repeat unit scission events and achieve high depolymerization conversions.34, 35, 41-43 In terms of RAFT polymers, previous work by Moad and co-workers showed the possibility of degradation at high temperatures (>300 °C).41 Although the focus of this work was to study the thermolysis of the RAFT end-groups, rather than to promote depolymerization, it provided important preliminary evidence that macromonomer formation is possible at elevated temperatures.41-45 For ATRP polymers, high temperatures were also required for depropagating radicals to be generated and lactonization was cited as the primary side reaction preventing low temperature depolymerization.34, 35, 46 As such, in both cases the bulk depolymerization of either RAFT or ATRP materials at lower temperatures was not possible. Inspired by previous work by Moad and co-workers we envisaged that macromonomer formation, either produced in situ or through a facile end-group modification strategy, could promote the chain-end low temperature bulk depolymerization of both RAFT and ATRP polymers while significantly minimizing the contribution from scission events.41, 45, 47, 48
In this work, we first trigger the solvent-free depolymerization of RAFT polymers at 200 °C and provide unambiguous evidence that the partial macromonomer formation is primarily responsible for the high depolymerization conversions obtained. Through an efficient chain-end depolymerization approach at lower temperatures, high temperature scission events and side reactions can be avoided, therefore suppressing monomer waste (i.e. sacrificial monomer that is essential to trigger scission) and recovering high amounts of pristine monomer (Scheme 1).49-57 To aid a more complete macromonomer formation, we then performed a facile and near quantitative post-polymerization modification of the ATRP and RAFT polymers. The modified polymers were subsequently successfully depolymerized resulting in up to 90 % monomer regeneration at temperatures as low as 220 °C. The scope of our methodology was expanded to include various molecular weights and dispersities, a number of RAFT agents (e.g. dithiobenzoate, trithiocarbonate, dithiocarbamate) and a selection of polymethacrylates with different repeat units (e.g. poly(methyl methacrylate), poly(butyl methacrylate), and poly(trifluoroethyl methacrylate)). The potential to scale up the developed methodology was also explored to exemplify the robustness of our approach.
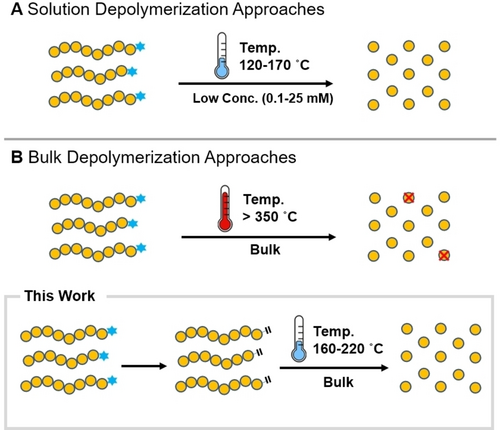
Depolymerization approaches for polymers obtained by controlled radical polymerization. Advantages and disadvantages of these methods are illustrated.
Results and Discussion
To investigate the bulk depolymerization of ATRP and RAFT polymers, PMMA was synthesized by photoATRP and thermal RAFT polymerization, respectively, followed by rigorous precipitation to remove residual monomer.58-61 The purified polymers exhibited comparable molecular weights and dispersity (Figures S1–5), thus allowing for a fair comparison between their depolymerization profiles, as recorded by thermogravimetric analysis (TGA, Figure S6). For the ATRP polymer, the main onset of depolymerization was observed at temperatures just above 350 °C, with 400 °C required for complete weight loss of the polymer (Figure 1a, blue traces). It is noted that a very small amount of depolymerization was possible at lower temperatures as evidenced by 1H nuclear magnetic resonance (NMR) spectroscopy and TGA but the vast majority of the polymer chains did not depolymerize until very high temperatures had been reached (Figures 1a–1b, blue). The high temperatures required here suggest that the polymer synthesized by ATRP can only depolymerize once side group or backbone scission events have occurred, with repeat unit degradation essential in generating the backbone radicals that can partially unzip the polymer chain.50, 62, 63 To verify this hypothesis, the depolymerization of a polymer without reactive or labile end-groups (prepared by anionic polymerization), was also studied as a reference (Figures 1a–1b, green). Under otherwise identical conditions, significant weight loss only occurred at temperatures higher than 350 °C, with no monomer detected by 1H NMR when a sample was taken at 220 °C. Altogether, these results suggest that the main depolymerization mechanism for both ATRP and anionic polymers is nearly identical, and although ATRP materials may still generate a small amount of monomer at lower temperatures, the carbon-bromide bond is not sufficiently thermally labile to trigger efficient depolymerization.
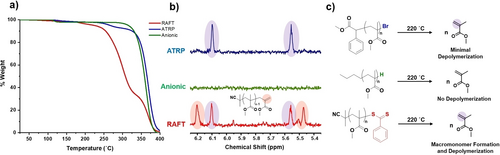
The depolymerization of PMMA prepared by ATRP, RAFT and anionic polymerization. In a), TGA traces are presented to illustrate the depolymerization profile of these various polymers. Experiments were performed with a heating rate of 1 °C per minute. In b) zoomed in 1H NMR spectra of each polymer's vinyl region are shown after heating to 220 °C. MMA monomer and macromonomer peaks are highlighted in purple and orange, respectively. In c) Schemes are shown to illustrate the relative extent of low-temperature depolymerization after heating these polymers to 220 °C.
In stark contrast, the depolymerization of the RAFT polymer (PMMA-dithiobenzoate (DTB)) presented a completely different profile (Figures 1a–1b, red). Initial weight loss was recorded at ≈200 °C followed by a significant reduction in weight commencing at 250 °C while approximately 50 % of depolymerization took place by 300 °C. This contrasts previous reports where all depolymerization occurred at temperatures greater than 300 °C.41 We attributed this difference to the slower heating rate used for our experiments (1 °C/minute vs 5 or 10 °C/minute) resulting in either a change in the depolymerization pathway or allowing more time for suitable depropagating radicals to form (Figure S7). The significantly lower onset for the RAFT polymer (100 °C earlier than its anionic counterpart) was hypothesized to be due to a rapid unzipping chain-end depolymerization reaction, rather than random scission events. To further investigate our hypothesis, samples were taken periodically throughout the RAFT depolymerization and were subsequently analyzed by both size exclusion chromatography (SEC) and 1H NMR spectroscopy. On measuring SEC from a sample obtained at 220 °C, a 95 % decrease in the polymer signal was observed in the UV detector, illustrating that the RAFT agent had been near-quantitatively degraded (Figures S8–S10). Upon analysis of the same sample by 1H NMR, a significant amount of recovered monomer was detected, accompanied by an additional set of distinctive vinyl peaks at 5.45 and 6.20 ppm, which we attributed to macromonomer formation. This was in line with previous reports proposing Chugaev elimination as a potential end-group degradation pathway (Scheme S1, Figure 1).41, 43 In total, 1H NMR showed that 65 % of the polymer chains had been converted to macromonomer which closely matched the percentage of weight loss in TGA by 325 °C (66 %), thus suggesting that the macromonomer formation may be responsible for the observed lower temperature depolymerization of RAFT polymers (Figure 1, red). To provide further evidence, we subsequently sampled the reaction at 325 °C (the temperature at which low temperature depolymerization ceased) and 1H NMR confirmed the complete consumption of the macromonomer (Figure S11). In addition, SEC analysis from the sample obtained at 325 °C showed that the shape of the molecular weight distribution was nearly identical to the starting polymer, thus further validating our hypothesis of a lower temperature rapid chain-end radical unzipping (Figure S12). Should side-group scission have been involved, a decrease in Mn would instead be expected (e.g. usually observed by low molecular weight tailing of the molecular weight distribution), as is the case of the anionic polymer whereby a lower Mn was obtained (7400 vs 9400) accompanied with an increase in dispersity (1.25 vs 1.09) when sampling at 360 °C (Figure S13).53 In the case of the RAFT polymer, the remaining one third of chains underwent weight loss at high temperatures (350 °C), similar to those at which the anionic and ATRP polymer depolymerized, thus illustrating that high-temperature repeat unit scission was essential to achieve high depolymerization conversions in all cases (Scheme S2 & Figure S14). In a similar fashion, when trithiocarbonate (TTC) and chloropyrazole dithiocarbamate (CPy) terminated-RAFT polymers (Scheme S3) were depolymerized, a significant contribution from scission was also observed (Figure S15). It is noted that depolymerization through scission not only occurs at significantly higher temperatures but also partially degrades the monomer. As such, facilitating lower temperature depolymerization for the vast majority of the chains, would result in a higher purity monomer generation.
Considering the importance of the macromonomer formation, we envisaged that by converting the ATRP and RAFT polymer end-groups to double bonds, we could potentially trigger a more efficient bulk depolymerization. The advantages of such transformation would be to (i) enhance the depolymerization conversion, (ii) retrieve pristine monomer by avoiding scission events and RAFT chain-end contaminants, and (iii) significantly lower the bulk depolymerization temperature. To explore the feasibility and the benefits of this scenario, we sought a polymerization method whereby macromonomer would be the sole polymerization product. Inspired by previous work from Manring and co-workers, our attention was directed to catalytic chain transfer polymerization, a method which can in principle result in highly pure PMMA macromonomer.47, 64-66 To maximize the macromonomer formation, we utilized CoBF (bis[(difluoroboryl)dimethylglyoximato]cobalt(II)) as the catalytic chain transfer agent and synthesized PMMA, (Mn=4100, Ð=1.26 after purification) which exhibited nearly quantitative end-group fidelity (i.e. macromonomer formation), as indicated by both SEC and MALDI-ToF-MS (Figures S16–18). The purified PMMA was then subjected to our previously optimized depolymerization conditions. Consistent with previous work,47 the depolymerization onset occurred at 150 °C with weight loss evidenced in the TGA chromatogram and 1H NMR clearly demonstrating monomer regeneration (Figures 2a & S19). This is a much lower onset temperature than the polymers prepared by RAFT, ATRP and anionic polymerization, with depolymerization observed at temperatures 200 °C lower than the anionic and ATRP polymers and actually ≈50 °C lower than that of the RAFT polymer. Following the early onset, rapid depolymerization was evident as the temperature exceeded 220 °C and a total of 92 % of depolymerization was reached by 260 °C (Figure 2a). This acceleration of the depolymerization was attributed to the more rapid removal of the monomer produced at these elevated temperatures. When comparing the depolymerization of the CCTP macromonomer to the polymers prepared by the other methods, the temperature required to achieve 90 % depolymerization was reduced by more than 125 °C in all cases (Figure 2b). To gain a better mechanistic understanding, we performed a detailed kinetic analysis in which individual samples were taken at various depolymerization conversions (18 %, 24 %, 32 %, 50 %, 68 % and 84 %, Table S1 & Figures S20–21). On measuring the SECs, the molecular weight distribution perfectly overlaps at all timepoints despite the high percentages of regenerated monomer as confirmed by TGA (Figures S21–22). In addition, 1H NMR analysis shows the gradual disappearance of the macromonomer peaks as the reaction progresses (Figures S23–24). Taken altogether, this data suggests a rapid unzipping mechanism whereby the polymer chain-end is first activated followed by a complete unzipping back to monomer in a single step. Our current hypothesis is that the olefin terminus can be activated to form the initiating chain-end radical through some type of self-initiation, similar to the auto-initiation of small monomers.67, 68 It is also not unlikely that radicals are formed at these high temperatures which can subsequently chain transfer to the macromonomer chain-end. However, the precise radical source required for this macromonomer activation is currently unclear.
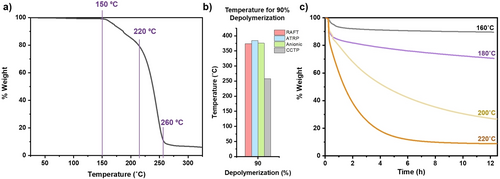
The depolymerization of PMMA obtained by CCTP. In a) a TGA trace is presented, which illustrates the depolymerization profile of this polymer. Experiments were performed with a heating rate of 1 °C per minute. The onsets of slow and rapid depolymerization and the temperature at which low-temperature depolymerization is complete are illustrated in purple. In b) a bar chart compares the temperatures at which 90 % depolymerization is achieved for polymers obtained from various methods. In c), TGA traces are presented, which illustrates the depolymerization profile of this polymer, when the temperature was increased by 10 °C/minute and then maintained at various temperatures for 12 hours.
To further lower the depolymerization temperature, a series of isothermal experiments were next performed where rather than continuously heating the polymer, the polymer was heated to a specific temperature, which was then maintained for a selected period of time. These experiments allow us to observe the maximum possible depolymerization at any given temperature. The first isothermal experiment of the CCTP polymer at 160 °C (Figure 2c, black) resulted in approximately 10 % of mass loss after 12 hours and subsequent experiments at 180 °C, 200 °C and 220 °C, gave rise to 30 %, 73 % and 91 % of depolymerization respectively (Figure 2c, purple, yellow & orange & Figure S25). These data illustrate that a near-quantitative bulk depolymerization can be achieved at temperatures as low as 220 °C. However, thus far, all depolymerization reactions had been performed in a TGA, with very small quantities of polymer (≈7 mg) and the purity of the obtained monomer had not been assessed. A bulk vacuum distillation experiment was performed by heating 0.5 grams of polymer to 220 °C. Notably, just over 87 % mass loss was observed in just 4 hours with over 0.4 g of monomer obtained (408 mg), an impressive 81.6 % yield. Importantly, on 1H NMR analysis, methyl methacrylate monomer was clearly observed in very high purity (Figure S26). The high purity of the recovered monomer was attributed to the lower temperature depolymerization whereby the contribution from scission events had presumably been eliminated.
Collectively, these experiments highlight that if we can successfully convert ATRP and RAFT polymers to macromonomers, a much lower depolymerization temperature could be employed (Figure 3a). In addition, another advantage of this approach would be the possibility to obtain pristine monomer while suppressing the side reactions that occur at much higher temperatures. For instance, a macromonomer end-group would significantly lower the depolymerization temperature of ATRP-synthesized materials. In the case of RAFT polymers, an efficient modification would also bypass RAFT chain-end degradation and the resulting contaminants. Such a transformation would also create the prospect of depolymerizing a far wider range of materials with controlled molecular weights, dispersity and architecture.69-71 This is in stark contrast to polymers made by free radical polymerization which are not only narrow in scope but can undergo only a small degree of depolymerization (approximately 30 %) as they contain just a very limited macromonomer percentage.72, 73

The effect of converting ATRP (left panels) and RAFT (right panels) polymers to macromonomer on the extent of their respective depolymerization. In a) a Scheme illustrates the formation of macromonomer and the subsequent depolymerization. In b) and c) TGA traces are presented with experiments performed using a heating rate of 1 °C per minute, and in d) and e) TGA traces are presented with experiments performed at a constant temperature of 220 °C for 12 hours after heating at 10 °C/minute.
To modify the ATRP bromine end-group, MMA monomer, ATRP photocatalyst and CoBF were added together with PMMA-Br in anisole with the following ratio: [PMMA-Br] : [MMA] : [FeBr3/TBABr] : [CoBF] of 1 : 5 : 0.15 : 0.04 and the mixture was left to stir overnight under blue light irradiation. 1H NMR analysis confirmed a near-quantitative macromonomer formation (>90 %) and the purified polymer was subsequently subjected to depolymerization (Figure S27). It is noted that our developed conditions resulted in a higher percentage of macromonomer conversion when compared to previous reports.74 This was of particular importance for our study, as the percentage of macromonomer formation determines the maximum extent of depolymerization possible. TGA revealed an onset of depolymerization at 160 °C for the modified ATRP polymer, a remarkably lower onset when compared to the Br-terminated analog which required temperatures higher than 350 °C to record a significant extent of depolymerization. A near-quantitative depolymerization was achieved at 270 °C with depolymerization conversions exceeding 90 % (Figure 3b). The key to the success of this approach is the vinyl end-group installed by the successful transformation to macromonomer. Thanks to this, monomer is generated at very low (for bulk depolymerization) temperatures and is constantly removed from the reaction as a gas, preventing the equilibrium monomer concentration from being reached and allowing depolymerization to proceed to high conversions at temperatures below the ceiling temperature. By having a consistent end-group on all the polymer chains, not only can we generate all the monomer from a given polymer chain, we can also generate monomer from almost all of the chains, thus allowing high overall yields to be obtained. This depolymerization approach is therefore far more energy efficient to perform, not only avoiding the necessity for high temperature scission reactions to trigger monomer generation, but also circumventing the requirement of high polymer dilutions to lower the ceiling temperature and prevent the equilibrium monomer concentration from being surpassed.75-77 Importantly, the reaction could be conducted without solvent or catalyst, instead with just the polymer as the only component, so is therefore very simple to carry out, avoiding the need to optimize reaction conditions. To investigate the compatibility of our approach with other polymers, we subsequently synthesized poly(butyl methacrylate). The Br chain-end was then converted to macromonomer and TGA confirmed a successful lower temperature depolymerization yielding high monomer regeneration, in line with the PMMA macromonomer (Figures S28–S30). To summarize, through an efficient macromonomer transformation strategy, ATRP polymers were shown to depolymerize in bulk at significantly lower temperatures.
Considering the success of these experiments, we envisaged that we could further leverage this macromonomer transformation strategy to also lower the depolymerization temperatures of RAFT-synthesized polymers. By following a similar transformation methodology to the ATRP analogs, the DTB end-group of PMMA produced by RAFT polymerization was modified under the optimal reaction conditions: [PMMA-DTB] : [MMA] : [AIBN] : [CoBF] of 1 : 5 : 0.08 : 0.15 at 80 °C in acetonitrile achieving approximately 90 % of macromonomer formation (Figures S31–S32).78 Similarly, after purification, a depolymerization experiment was performed (Figures 3c). Interestingly, the RAFT macromonomer had a much faster depolymerization than the original RAFT polymer. In addition, higher depolymerization conversions at lower temperatures could also be achieved with 90 % weight loss for the RAFT macromonomer as opposed to 66 % weight loss for the original RAFT (DTB) polymer. The difference in rate was attributed to the additional step required to form the macromonomer for the initial RAFT polymer, the presence of degraded thiol species in the depolymerization mixture or the occurrence of a potential side reaction, i.e. backbiting or radical chain transfer. These side reactions could be of particular importance, as one-third of the original RAFT polymer chains did not form macromonomer on heating, but instead formed a thermally stable end-group and only depolymerized through a much higher temperature side group or backbone-triggered scission pathway. Instead, by converting the RAFT polymer to macromonomer, this side-reaction was avoided, thus resulting in a lower temperature depolymerization and up to 90 % of depolymerization conversion. At this point, we were interested in probing the potential of our method to operate with different molecular weights and dispersities. In particular, two additional RAFT polymers were synthesized with an Mn of 6100 and 23500 and were exposed to depolymerization upon successful end-group modification to macromonomer (Figures S33–35). Regardless of the molecular weight, a lower temperature depolymerization was observed regenerating a high percentage of monomer. The slightly lower depolymerization temperature observed for the lower molecular weight polymers may be due to the lower molecular weight sample containing a greater amount of terminal vinyl groups, leading to a more rapid initiation process. In addition, our method could be applied to polymers of higher dispersity (i.e. 1.49), which exhibited a slightly lower onset of depolymerization and slower rate (Figures S36–37). This is due to the much greater range of molecular weight chains within the high dispersity polymer. We also studied the depolymerization of poly(trifluoroethyl methacrylate) (PTFEMA) macromonomer which was obtained by modifying PTFEMA-DTB. This resulted in a lower temperature depolymerization, albeit to a lesser extent when compared to the PMMA analog (Figures S38–39). Last but not least, we synthesized PMMA using trithiocarbonate and dithiocarbamate chloropyrazole as the RAFT agents (Figures S40–42). In both cases, the end-groups were successfully converted to macromonomers which then also proceeded to undergo a lower temperature depolymerization (Figure S43). However, the extent of depolymerization did not exceed 85 % and as such DTB is still the recommended RAFT agent if higher conversions are desired.
Isothermal experiments for both the ATRP and RAFT derived macromonomers were subsequently conducted (Figures 3d–e). After 12 hours at 220 °C, 85–90 % depolymerization of the macromonomers could be achieved in both cases. This compares favorably to both of the precursor polymers, where it was only possible to achieve 8 % and 27 % under otherwise identical conditions, further emphasizing the success of our approach. To achieve more than 90 % depolymerization either a gradual temperature ramp to 260 °C is required, or the temperature can be reduced to 220 °C when longer reaction times are utilized. Together, these temperatures are significantly lower than those reported for random repeat unit scission so in both cases there is a vast improvement in comparison to their unmodified precursors, where around 400 °C was required to achieve full depolymerization.
Finally, a further advantage of bulk depolymerization that we wanted to fully exploit is the ability to scale up the reactions (Figure 4a) and obtain pristine monomer considering that scission events can be eliminated at our lower depolymerization temperatures. A particularly challenging task for solution approaches is the low concentrations at which they are performed, for example, to successfully depolymerize 5 g of polymer at 5 mM repeat unit concentration, 10 litres of solvent would be required.37 This makes the scale-up of these depolymerization processes unfeasible. Alternative scalable approaches in bulk, have only been reported at temperatures greater than 400 °C.49-52 To address this, a higher scale vacuum distillation at 220 °C was performed starting with a 10 g of an ATRP-derived macromonomer (Figures 4b–d & S44–46). It is noted that such a simple distillation set-up is only possible due to the low depolymerization temperatures employed. After 4 hours of reaction, 8.4 g of monomer could be regenerated (84 % yield) and the retrieved MMA was obtained in a high purity. The collected monomer was then employed, without any further purification, to synthesize another batch of both ATRP and RAFT polymer with controlled molecular weight and dispersity, further illustrating the closed-loop nature of our approach (Figures 4c–d & S47). Together this exemplifies the success of our strategy, with low-temperature high-scale depolymerization being attainable, while generating monomer in high yields.
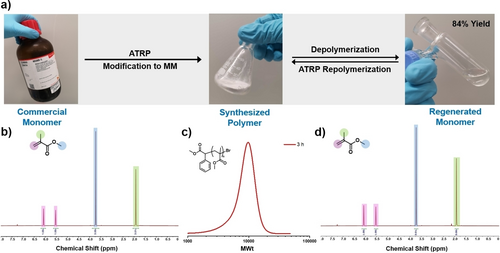
The closed-loop and scalable depolymerization of PMMA obtained from ATRP. In a), photographs show the commercial monomer being polymerized and modified into macromonomer, followed by the depolymerization of this polymer regenerating the starting monomer. In b), a 1H NMR spectrum of commercial monomer is presented, in c) SEC chromatogram illustrating a polymer produced from regenerated monomer, and in d) a 1H NMR of regenerated monomer is shown.
Conclusion
In this work we showed that through an efficient end-group modification strategy, polymers synthesized by either ATRP or RAFT polymerizations can undergo a low temperature bulk depolymerization with onset temperatures at as low as 150 °C. Notably, very high depolymerization conversions exceeding 90 % could be achieved on a short time-scale. Our approach proceeds in the complete absence of solvents, without requiring additional catalysts or components and can regenerate high purity monomer in a very high yield. Scalability of our methodology to up to 10 grams using a simple vacuum distillation setup was also demonstrated thus highlighting the robustness of this strategy. The recovered pristine monomer was subsequently employed for another polymerization cycle resulting in the synthesis of well-defined polymers with low dispersity. This is a potentially important step in developing scalable and more energy efficient depolymerization and generates many future new research directions for chemical recycling.
Acknowledgments
A.A. gratefully acknowledges ETH Zurich (Switzerland) and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (DEPO: Grant No. 949219) for financial support. N.P.T. acknowledges the award of a DECRA Fellowship from the ARC (DE180100076). We acknowledge Professor Markus Niederberger and Ms. Aina Perappadan (Laboratory for Multifunctional Materials) for access to the TGA instrument, and Dr. Louis Bertschi and Mr. Daniel Wirz (MoBias) for access to the MALDI-ToF Mass Spectrometer. Open Access funding provided by Eidgenössische Technische Hochschule Zürich.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.