Turning on Asymmetric Catalysis of Achiral Metal-Organic Frameworks by Imparting Chiral Microenvironment
Ge Yang
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorWenwen Shi
CAS Key Laboratory of Precision and Intelligent Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Yunyang Qian
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao Zheng
CAS Key Laboratory of Precision and Intelligent Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
Department of Chemistry, Fudan University, 200433 Shanghai, P. R. China
Search for more papers by this authorProf. Dr. Zheng Meng
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hai-Long Jiang
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
Search for more papers by this authorGe Yang
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorWenwen Shi
CAS Key Laboratory of Precision and Intelligent Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Yunyang Qian
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao Zheng
CAS Key Laboratory of Precision and Intelligent Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
Department of Chemistry, Fudan University, 200433 Shanghai, P. R. China
Search for more papers by this authorProf. Dr. Zheng Meng
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hai-Long Jiang
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 230026 Hefei, Anhui, P. R. China
Search for more papers by this authorGraphical Abstract
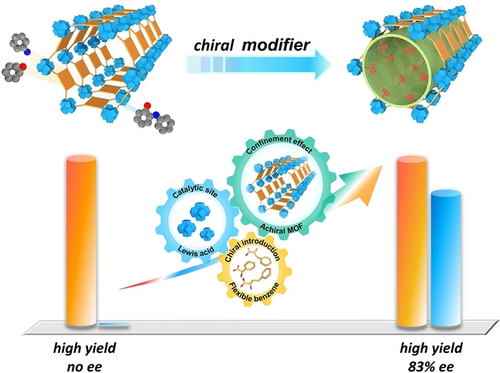
The chiral microenvironment around catalytically active metal clusters in a MOF, PCN-222(Cu), is created by simply grafting chiral molecules (R)−Cn−COOH (n=1, 2, 3). Owing to multi-level modulation such as hydrogen interaction, steric hindrance, and confinement effect caused by the microenvironment, the resulting (R)−Cn@PCN-222(Cu) exhibits high activity and enantioselectivity in the asymmetric ring-opening of cyclohexene oxide with aniline.
Abstract
The development of heterogeneous asymmetric catalysts has attracted increasing interest in synthetic chemistry but mostly relies on the immobilization of homogeneous chiral catalysts. Herein, a series of chiral metal–organic frameworks (MOFs) have been fabricated by anchoring similar chiral hydroxylated molecules (catalytically inactive) with different lengths onto Zr-oxo clusters in achiral PCN-222(Cu). The resulting chiral MOFs exhibit regulated enantioselectivity up to 83 % ee in the asymmetric ring-opening of cyclohexene oxide. The chiral molecules furnished onto the catalytic Lewis sites in the MOF create multilevel microenvironment, including the hydrogen interaction between the substrate and the chiral −OH group, the steric hindrance endowed by the benzene ring on the chiral molecules, and the proximity between the catalytic sites and chiral molecules confined in the MOF pores, which play crucial roles and synergistically promote chiral catalysis. This work nicely achieves heterogeneous enantioselective catalysis by chiral microenvironment modulation around Lewis acid sites.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202308089-sup-0001-misc_information.pdf5.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Akiyama, I. Ojima, in Catalytic Asymmetric Synthesis, Wiley-VCH, Weinheim, 2022.
10.1002/9781119736424 Google Scholar
- 2
- 2aT. P. Yoon, E. N. Jacobsen, Science 2003, 299, 1691–1693;
- 2bD. W. C. MacMillan, Nature 2008, 455, 304–308;
- 2cS. Shaw, J. D. White, Chem. Rev. 2019, 119, 9381–9426;
- 2dG. Yang, W. Zhang, Chem. Soc. Rev. 2018, 47, 1783–1810;
- 2eC. Portolani, G. Centonze, P. Righi, G. Bencivenni, Acc. Chem. Res. 2022, 55, 3551–3571.
- 3
- 3aD. Zhang, Z. Su, Q. He, Z. Wu, Y. Zhou, C. Pan, X. Liu, X. Feng, J. Am. Chem. Soc. 2020, 142, 15975–15985;
- 3bD. A. Kutateladze, C. C. Wagen, E. N. Jacobsen, J. Am. Chem. Soc. 2022, 144, 15812–15824;
- 3cL. Zhang, Z. Wang, Z. Han, K. Ding, Angew. Chem. Int. Ed. 2020, 59, 15565–15569.
- 4
- 4aM. B. Buendia, S. Kegnæs, S. Kramer, Adv. Synth. Catal. 2020, 362, 5506–5512;
- 4bY. Saito, S. Kobayashi, J. Am. Chem. Soc. 2020, 142, 16546–16551;
- 4cY. Saito, S. Kobayashi, Angew. Chem. Int. Ed. 2021, 60, 26566–26570.
- 5
- 5aH. Furukawa, K. E. Cordova, M. O'Keeffe, O. M. Yaghi, Science 2013, 341, 1230444;
- 5bR.-B. Lin, Z. Zhang, B. Chen, Acc. Chem. Res. 2021, 54, 3362–3376;
- 5cS. Horike, S. Kitagawa, Nat. Mater. 2022, 21, 983–985;
- 5dA. N. Hong, H. Yang, X. Bu, P. Feng, EnergyChem 2022, 4, 100080;
- 5eK.-Y. Wang, Z. Yang, J. Zhang, S. Banerjee, E. A. Joseph, Y.-C. Hsu, S. Yuan, L. Feng, H.-C. Zhou, Nat. Protoc. 2023, 18, 604–625;
- 5fY.-S. Wei, L. Zou, H.-F. Wang, Y. Wang, Q. Xu, Adv. Energy Mater. 2022, 12, 2003970;
- 5gM.-S. Yao, W.-H. Li, G. Xu, Coord. Chem. Rev. 2021, 426, 213479.
- 6
- 6aG. Li, S. Zhao, Y. Zhang, Z. Tang, Adv. Mater. 2018, 30, 1800702;
- 6bJ. Liu, T. A. Goetjen, Q. Wang, J. G. Knapp, M. C. Wasson, Y. Yang, Z. H. Syed, M. Delferro, J. M. Notestein, O. K. Farha, J. T. Hupp, Chem. Soc. Rev. 2022, 51, 1045–1097;
- 6cL. Zeng, X. Guo, C. He, C. Duan, ACS Catal. 2016, 6, 7935–7947;
- 6dY.-B. Huang, J. Liang, X.-S. Wang, R. Cao, Chem. Soc. Rev. 2017, 46, 126–157;
- 6eS. Navalón, A. Dhakshinamoorthy, M. Álvaro, B. Ferrer, H. García, Chem. Rev. 2023, 123, 445–490;
- 6fL. Jiao, J. Wang, H.-L. Jiang, Acc. Mater. Res. 2021, 2, 327–339;
- 6gY.-L. Yang, Y.-R. Wang, L.-Z. Dong, Q. Li, L. Zhang, J. Zhou, S.-N. Sun, H.-M. Ding, Y. Chen, S.-L. Li, Y.-Q. Lan, Adv. Mater. 2022, 34, 2206706;
- 6hC. Feng, Z.-P. Wu, K.-W. Huang, J. Ye, H. Zhang, Adv. Mater. 2022, 34, 2200180;
- 6iL. Li, Z. Li, W. Yang, Y. Huang, G. Huang, Q. Guan, Y. Dong, J. Lu, S.-H. Yu, H.-L. Jiang, Chem 2021, 7, 686–698;
- 6jL. Jiao, H.-L. Jiang, Chin. J. Catal. 2023, 45, 1–5.
- 7
- 7aZ. Xia, C. He, X. Wang, C. Duan, Nat. Commun. 2017, 8, 361;
- 7bW. Gong, X. Chen, W. Zhang, K. O. Kirlikovali, B. Nan, Z. Chen, R. Si, Y. Liu, O. K. Farha, Y. Cui, J. Am. Chem. Soc. 2022, 144, 3117–3126;
- 7cM. Pan, K. Wu, J.-H. Zhang, C.-Y. Su, Coord. Chem. Rev. 2019, 378, 333–349;
- 7dC. Kutzscher, H. C. Hoffmann, S. Krause, U. Stoeck, I. Senkovska, E. Brunner, S. Kaskel, Inorg. Chem. 2015, 54, 1003–1009;
- 7eL. Ma, J. M. Falkowski, C. Abney, W. Lin, Nat. Chem. 2010, 2, 838–846;
- 7fN. Antil, N. Akhtar, R. Newar, W. Begum, A. Kumar, M. Chauhan, K Manna, ACS Catal. 2021, 11, 10450–10459;
- 7gT.-Y. Zhou, B. Auer, S. J. Lee, S. G. Telfer, J. Am. Chem. Soc. 2019, 141, 1577–1582;
- 7hY. Zhang, S. Chen, A.-M. Al-Enizi, A. Nafady, Z. Tang, S. Ma, Angew. Chem. Int. Ed. 2022, 62, e202213399;
- 7iR. E. Morris, X. Bu, Nat. Chem. 2010, 2, 353–361.
- 8
- 8aK. D. Nguyen, C. Kutzscher, F. Drache, I. Senkovska, S Kaskel, Inorg. Chem. 2018, 57, 1483–1489;
- 8bM. Banerjee, S. Das, M. Yoon, H. J. Choi, M. H. Hyun, S. M. Park, G. Geo, K. Kim, J. Am. Chem. Soc. 2009, 131, 7524–7525.
- 9
- 9aS. J. Benkovic, S. Hammes-Schiffer, Science 2003, 301, 1196–1202;
- 9bY.-P. Xue, C.-H. Cao, Y.-G. Zheng, Chem. Soc. Rev. 2018, 47, 1516–1561;
- 9cJ. Wu, X. Guan, Z. Dai, R. He, X. Ding, L. Yang, G. Ge, Coord. Chem. Rev. 2021, 427, 213600.
- 10
- 10aQ. Fu, X. Bao, Nat. Catal. 2019, 2, 834–836;
- 10bJ. C. Fontecilla-Camps, A. Volbeda, Chem. Rev. 2022, 122, 12110–12131;
- 10cJ. Wang, C. D. Buchman, J. Seetharaman, D. J. Miller, A. D. Huber, J. Wu, S. C. Chai, E. Garcia-Maldonado, W. C. Wright, J. Chenge, T. Chen, J. Am. Chem. Soc. 2021, 143, 18467–18480;
- 10dJ. P. López-Alonso, M. Lázaro, D. Gil-Cartón, P. H. Choi, L. Tong, M. Valle, Nat. Commun. 2022, 13, 6185.
- 11
- 11aB. Hou, S. Yang, K. Yang, X. Han, X. Tang, Y. Liu, J. Jiang, Y. Cui, Angew. Chem. Int. Ed. 2021, 60, 6086–6093;
- 11bH.-C. Ma, C.-C. Zhao, G.-J. Chen, Y.-B. Dong, Nat. Commun. 2019, 10, 3368;
- 11cJ. Jiao, J. Dong, Y. Li, Y. Cui, Angew. Chem. Int. Ed. 2021, 60, 16568–16575.
- 12
- 12aD. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei, H.-C. Zhou, Angew. Chem. Int. Ed. 2012, 51, 10307–10310;
- 12bW. Morris, B. Volosskiy, S. Demir, F. Gandara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart, O. M. Yaghi, Inorg. Chem. 2012, 51, 6443–6445;
- 12cY. Chen, T. Hoang, S. Ma, Inorg. Chem. 2012, 51, 12600–12602.
- 13
- 13aP. Deria, J. E. Mondloch, E. Tylianakis, P. Ghosh, W. Bury, R. Q. Snurr, J. T. Hupp, O. K. Farha, J. Am. Chem. Soc. 2013, 135, 16801–16804;
- 13bX. Ma, H. Liu, W. Yang, G. Mao, L. Zheng, H.-L. Jiang, J. Am. Chem. Soc. 2021, 143, 12220–12229.
- 14
- 14aC. Wang, L. Luo, H. Yamamoto, Acc. Chem. Res. 2016, 49, 193–204;
- 14bH. Bao, J. Wu, H. Li, Z. Wang, T. You, K. Ding, Eur. J. Org. Chem. 2010, 6722–6726;
- 14cN. Deshpande, A. Parulkar, R. Joshi, B. Diep, A. Kulkarni, N. A. Brunelli, J. Catal. 2019, 370, 46–54.
- 15J. Lyu, X. Zhang, P. Li, X. Wang, C. T. Buru, P. Bai, X. Guo, O. K. Farha, Chem. Mater. 2019, 31, 4166–4172.
- 16
- 16aT. Hansen, P. Vermeeren, R. Yoshisada, D. V. Filippov, G. A. van der Marel, J. D. C. Codée, T. A. Hamlin, J. Org. Chem. 2021, 86, 3565–3573;
- 16bK. Doitomi, K. Xu, H. Hirao, Dalton Trans. 2017, 46, 3470–3481.
- 17
- 17aT. Lu, F. Chen, J. Comput. Chem. 2012, 33, 580–592;
- 17bT. Lu, Q. Chen, J. Comput. Chem. 2022, 43, 539–555.