Accelerating Ethanol Complete Electrooxidation via Introducing Ethylene as the Precursor for the C−C Bond Splitting
Corresponding Author
Dr. Teng Chen
Air Force Logistics Academy, Xuzhou, Jiangsu, 221000 China
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorShen Xu
School of Biological and Chemical Engineering, Nanyang Institute of Technology, Nanyang, 473004 China
Search for more papers by this authorTaotao Zhao
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorXiaohang Zhou
Air Force Logistics Academy, Xuzhou, Jiangsu, 221000 China
Search for more papers by this authorCorresponding Author
Prof. Jianqiang Hu
Air Force Logistics Academy, Xuzhou, Jiangsu, 221000 China
Search for more papers by this authorXin Xu
Air Force Logistics Academy, Xuzhou, Jiangsu, 221000 China
Search for more papers by this authorChenjia Liang
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Min Liu
State Key Laboratory of Powder Metallurgy, School of Physical and Electronics, Central South University, Changsha, Hunan, 410083 China
Search for more papers by this authorCorresponding Author
Prof. Weiping Ding
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorCorresponding Author
Dr. Teng Chen
Air Force Logistics Academy, Xuzhou, Jiangsu, 221000 China
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorShen Xu
School of Biological and Chemical Engineering, Nanyang Institute of Technology, Nanyang, 473004 China
Search for more papers by this authorTaotao Zhao
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorXiaohang Zhou
Air Force Logistics Academy, Xuzhou, Jiangsu, 221000 China
Search for more papers by this authorCorresponding Author
Prof. Jianqiang Hu
Air Force Logistics Academy, Xuzhou, Jiangsu, 221000 China
Search for more papers by this authorXin Xu
Air Force Logistics Academy, Xuzhou, Jiangsu, 221000 China
Search for more papers by this authorChenjia Liang
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Min Liu
State Key Laboratory of Powder Metallurgy, School of Physical and Electronics, Central South University, Changsha, Hunan, 410083 China
Search for more papers by this authorCorresponding Author
Prof. Weiping Ding
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorGraphical Abstract
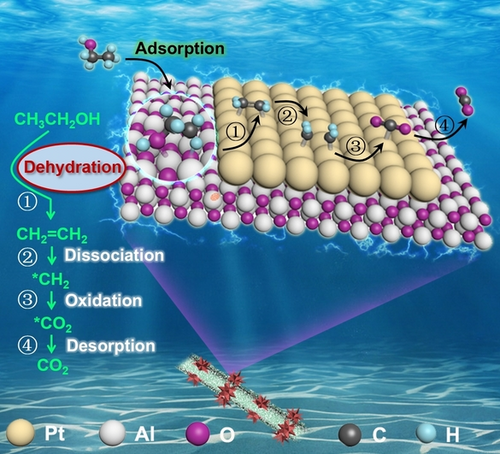
A unique ethylene-mediated pathway with a 100 % C1-selectivity for ethanol oxidation reaction (EOR) is proposed for the first time based on a well-structured Pt/Al2O3@TiAl catalyst, in which Al2O3 is responsible for generating ethylene from ethanol dehydration while Pt boosts the complete oxidation of ethylene to CO2.
Abstract
The crucial issue restricting the application of direct ethanol fuel cells (DEFCs) is the incomplete and sluggish electrooxidation of ethanol due to the chemically stable C−C bond thereof. Herein, a unique ethylene-mediated pathway with a 100 % C1-selectivity for ethanol oxidation reaction (EOR) is proposed for the first time based on a well-structured Pt/Al2O3@TiAl catalyst with cascade active sites. The electrochemical in situ Fourier transform infrared spectroscopy (FTIR) and differential electrochemical mass spectrometry (DEMS) analysis disclose that ethanol is primarily dehydrated on the surface of Al2O3@TiAl and the derived ethylene is further oxidized completely on nanostructured Pt. X-ray absorption and density functional theory (DFT) studies disclose the Al component doped in Pt nanocrystals can promote the EOR kinetics by lowering the reaction energy barriers and eliminating the poisonous species. Strikingly, Pt/Al2O3@TiAl exhibits a specific activity of 3.83 mA cm−2Pt, 7.4 times higher than that of commercial Pt/C and superior long-term durability.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202308057-sup-0001-misc_information.pdf2.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Chang, G. Wang, M. Wang, Q. Wang, B. Li, H. Zhou, Y. Zhu, W. Zhang, M. Omer, N. Orlovskaya, Q. Ma, M. Gu, Z. Feng, G. Wang, Y. Yang, Nat. Energy 2021, 6, 1144–1153;
- 1bY. Wang, M. Zheng, Y. Li, C. Ye, J. Chen, J. Ye, Q. Zhang, J. Li, Z. Zhou, X. Fu, J. Wang, S. Sun, D. Wang, Angew. Chem. Int. Ed. 2022, 61, e202115735;
- 1cK. Liu, W. Wang, P. Guo, J. Ye, Y. Wang, P. Li, Z. Lyu, Y. Geng, M. Liu, S. Xie, Adv. Funct. Mater. 2019, 29, 1806300.
- 2
- 2aB. Zhang, T. Sheng, Y. Wang, X. Qu, J. Zhang, Z. Zhang, H. Liao, F. Zhu, S. Dou, Y. Jiang, S. Sun, ACS Catal. 2017, 7, 892–895;
- 2bG. Yang, Q. Zhang, H. Yu, F. Peng, Particuology 2021, 58, 169–186.
- 3
- 3aS. Bai, Y. Xu, K. Cao, X. Huang, Adv. Mater. 2021, 33, 2005767;
- 3bY. Fang, S. Guo, D. Cao, G. Zhang, Q. Wang, Y. Chen, P. Cui, S. Cheng, W. Zuo, Nano Res. 2022, 15, 3933–3939.
- 4
- 4aC. Zhu, B. Lan, R. Wei, C. Wang, Y. Yang, ACS Catal. 2019, 9, 4046–4053;
- 4bF. Lv, W. Zhang, M. Sun, F. Lin, T. Wu, P. Zhou, W. Yang, P. Gao, B. Huang, S. Guo, Adv. Energy Mater. 2021, 11, 2100187.
- 5
- 5aQ. Chang, Y. Hong, H. J. Lee, J. H. Lee, D. Ologunagba, Z. Liang, J. Kim, M. J. Kim, J. W. Hong, L. Song, S. Kattel, Z. Chen, J. G. Chen, S−Il Choi, Proc. Natl. Acad. Sci. USA 2022, 119, e2112109119;
- 5bP. L. Kress, S. Zhang, Y. Wang, V. Çınar, C. M. Friend, E. C. H. Sykes, M. M. Montemore, J. Am. Chem. Soc. 2023, 145, 8401–8407;
- 5cJ. Tang, N. Tian, L. Xiao, Q. Chen, Q. Wang, Z. Zhou, S. Sun, J. Mater. Chem. A 2022, 10, 10902–10908;
- 5dM. Qiao, F. Meng, H. Wu, Y. Wei, X. Zeng, J. Wang, Small 2022, 18, 2204720;
- 5eQ. Chang, S. Kattel, X. Li, Z. Liang, B. M. Tackett, S. R. Denny, P. Zhang, D. Su, J. G. Chen, Z. Chen, ACS Catal. 2019, 9, 7618–7625;
- 5fY. Chen, J. Pei, Z. Chen, A. Li, S. Ji, H. Rong, Q. Xu, T. Wang, A. Zhang, H. Tang, J. Zhu, X. Han, Z. Zhuang, G. Zhou, D. Wang, Nano Lett. 2022, 22, 7563–7571.
- 6
- 6aC. Jiang, K. Hara, A. Fukuoka, Angew. Chem. Int. Ed. 2013, 52, 6265–6268;
- 6bR. Miyazaki, N. Nakatani, S. V. Levchenko, T. Yokoya, K. Nakajima, K. Hara, A. Fukuoka, J. Hasegawa, J. Phys. Chem. C 2019, 123, 12706–12715.
- 7P. Kostestkyy, J. Yu, R. J. Gorte, G. Mpourmpakis, Catal. Sci. Technol. 2014, 4, 3861–3869.
- 8
- 8aM. Li, Z. Zhao, W. Zhang, M. Luo, L. Tao, Y. Sun, Z. Xia, Y. Chao, K. Yin, Q. Zhang, L. Gu, W. Yang, Y. Yu, G. Lu, S. Guo, Adv. Mater. 2021, 33, 2103762;
- 8bL. Lai, G. Yang, Q. Zhang, H. Yu, F. Peng, J. Power Sources 2021, 509, 230397.
- 9
- 9aS. Jha, S. Mehta, Y. Chen, L. Ma, P. Renner, D. Y. Parkinson, H. Liang, ACS Sustainable Chem. Eng. 2020, 8, 9597–9598;
- 9bK. Bhattacharyya, S. Varma, A. K. Tripathi, S. R. Bharadwaj, A. K. Tyagi, J. Phys. Chem. B 2009, 113, 5917–5928.
- 10X. Kai, R. Li, T. Yang, S. Shen, Q. Ji, T. Zhang, Energy Convers. Manage. 2017, 146, 20–33.
- 11
- 11aY. Yang, J. Ren, Q. Li, Z. Zhou, S. Sun, W. Cai, ACS Catal. 2014, 4, 798–803;
- 11bS. Chen, T. Luo, K. Chen, Y. Lin, J. Fu, K. Liu, C. Cai, Q. Wang, H. Li, X. Li, J. Hu, H. Li, M. Zhu, M. Liu, Angew. Chem. Int. Ed. 2021, 60, 16607–16614;
- 11cX. Zhou, Y. Ma, Y. Ge, S. Zhu, Y. Cui, B. Chen, L. Liao, Q. Yun, Z. He, H. Long, L. Li, B. Huang, Q. Luo, L. Zhai, X. Wang, L. Bai, G. Wang, Z. Guan, Y. Chen, C. S. Lee, J. Wang, C. Ling, M. Shao, Z. Fan, H. Zhang, J. Am. Chem. Soc. 2022, 144, 547–555.
- 12A. Bach Delpeuch, F. Maillard, M. Chatenet, P. Soudant, C. Cremers, Appl. Catal. B 2016, 181, 672–680.
- 13
- 13aC. Li, X. Chen, L. Zhang, S. Yan, A. Sharma, B. Zhao, A. Kumbhar, G. Zhou, J. Fang, Angew. Chem. Int. Ed. 2021, 60, 7675–7680;
- 13bJ. M. Kim, A. Jo, K. A. Lee, H. J. Han, Y. J. Kim, H. Y. Kim, G. R. Lee, M. Kim, Y. Park, Y. S. Kang, J. Jung, K. H. Chae, E. Lee, H. C. Ham, H. Ju, Y. S. Jung, J. Y. Kim, Sci. Adv. 2021, 7, eabe9083;
- 13cB. Guo, J. Kang, T. Zeng, H. Qu, S. Yu, H. Deng, J. Bai, Adv. Sci. 2022, 9, 2201751.
- 14Q. Liu, S. Zhang, J. Liao, K. Feng, Y. Zheng, B. G. Pollet, H. Li, J. Power Sources 2017, 355, 191–198.
- 15
- 15aY. Hu, M. Zhu, X. Luo, G. Wu, T. Chao, Y. Qu, F. Zhou, R. Sun, X. Han, H. Li, B. Jiang, Y. Wu, X. Hong, Angew. Chem. Int. Ed. 2021, 60, 6533–6538;
- 15bT. Chen, Y. Xu, D. Meng, X. Guo, Y. Zhu, L. Peng, J. Hu, W. Ding, J. Energy Chem. 2022, 71, 304–312;
- 15cT. Chen, Y. Xu, S. Guo, D. Wei, L. Peng, X. Guo, N. Xue, Y. Zhu, Z. Chen, B. Zhao, W. Ding, iScience 2019, 11, 388–397.
- 16
- 16aZ. Wu, P. Yang, Q. Li, W. Xiao, Z. Li, G. Xu, F. Liu, B. Jia, T. Ma, S. Feng, L. Wang, Angew. Chem. 2023, 135, e202300406;
- 16bG. Liu, P. Liu, D. Meng, T. Zhao, X. Qian, Q. He, X. Guo, J. Qi, L. Peng, N. Xue, Y. Zhu, J. Ma, Q. Wang, X. Liu, L. Chen, W. Ding, Nat. Commun. 2023, 14, 513;
- 16cC. Deng, X. Qian, M Lu, Q. Liu, T. Zhao, J. Yang, T.g Chen, L. Dong, Appl. Catal. B 2023, 333, 122791.
- 17C. Zhan, L. Bu, H. Sun, X. Huang, Z. Zhu, T. Yang, H. Ma, L. Li, Y. Wang, H. Geng, W. Wang, H. Zhu, C. Pao, Q. Shao, Z. Yang, W. Liu, Z. Xie, X. Huang, Angew. Chem. Int. Ed. 2023, 62, e202213783.
- 18
- 18aT. Chen, J. Ma, S. Chen, Y. Wei, C. Deng, J. Chen, J. Hu, W. Ding, Chem. Eng. J. 2021, 415, 129031;
- 18bH. Lv, L. Sun, Y. Wang, S. Liu, B. Liu, Adv. Mater. 2022, 34, 2203612;
- 18cN. Logeshwaran, I. R. Panneerselvam, S. Ramakrishnan, R. S. Kumar, A. R. Kim, Y. Wang, D. J. Yoo, Adv. Sci. 2022, 9, 2105344.
- 19
- 19aZ. Zhang, J. Liu, J. Wang, Q. Wang, Y. Wang, K. Wang, Z. Wang, M. Gu, Z. Tang, J. Lim, T. Zhao, F. Ciucci, Nat. Commun. 2021, 12, 5235;
- 19bF. Hu, L. Yang, Y. Jiang, C. Duan, X. Wang, L. Zeng, X. Lv, D. Duan, Q. Liu, T. Kong, J. Jiang, R. Long, Y. Xiong, Angew. Chem. Int. Ed. 2021, 60, 26122–26127;
- 19cD. Chen, R. Lu, R. Yu, Y. Dai, H. Zhao, D. Wu, P. Wang, J. Zhu, Z. Pu, L. Chen, J. Yu, S. Mu, Angew. Chem. Int. Ed. 2022, 61, e202208642.
- 20
- 20aM Bełtowska-Brzezinska, T. Łuczak, M. Mączka, H. Baltruschat, U. Müller, J. Electroanal. Chem. 2002, 519, 101–110;
- 20bA. Berná, A. Kuzume, E. Herrero, J. M. Feliu, Surf. Sci. 2008, 602, 84–94.
- 21
- 21aS. Chen, T. Luo, X. Li, K. Chen, J. Fu, K. Liu, C. Cai, Q. Wang, H. Li, Y. Chen, C. Ma, L. Zhu, Y. Lu, T. Chan, M. Zhu, E. Cortés, M. Liu, J. Am. Chem. Soc. 2022, 144, 14505–14516;
- 21bS. Chen, X. Li, C. W. Kao, T. Luo, K. Chen, J. Fu, C. Ma, H. Li, M. Li, T. Chan, M. Liu, Angew. Chem. Int. Ed. 2022, 61, e202206233.