Chiral PSiSi-Ligand Enabled Iridium-Catalyzed Atroposelective Intermolecular C−H Silylation
Dr. Bo Yang
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
These authors contributed equally to this work.
Search for more papers by this authorJihui Gao
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
These authors contributed equally to this work.
Search for more papers by this authorXingfa Tan
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
Search for more papers by this authorCorresponding Author
Dr. Yicong Ge
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chuan He
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
Search for more papers by this authorDr. Bo Yang
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
These authors contributed equally to this work.
Search for more papers by this authorJihui Gao
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
These authors contributed equally to this work.
Search for more papers by this authorXingfa Tan
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
Search for more papers by this authorCorresponding Author
Dr. Yicong Ge
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chuan He
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong 518055 China
Search for more papers by this authorGraphical Abstract
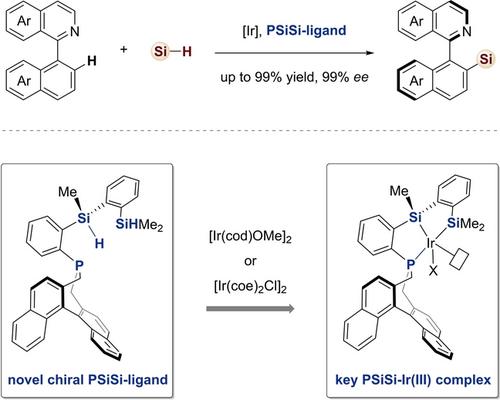
A new type of chiral pincer silyl ligand is developed, which enables an Iridium-catalyzed atroposelective intermolecular C−H silylation reaction. Key to the success of this transformation is the use of a novel chiral PSiSi-ligand, which facilitates the C−H silylation process with high chemical, regio- and stereo-control via a multi-coordinated silyl iridium complex.
Abstract
Catalytic enantioselective intermolecular C−H silylation offers an efficient approach for the rapid construction of chiral organosilicon compounds, but remains a significant challenge. Herein, a new type of chiral silyl ligand is developed, which enables the first iridium-catalyzed atroposelective intermolecular C−H silylation reaction of 2-arylisoquinolines. This protocol features mild reaction conditions, high atom economy, and remarkable yield with excellent stereoselectivity (up to 99 % yield, 99 % ee), delivering enantioenriched axially chiral silane platform molecules with facile convertibility. Key to the success of this unprecedented transformation relies on a novel chiral PSiSi-ligand, which facilitates the intermolecular C−H silylation process with perfect chem-, regio- and stereo-control via a multi-coordinated silyl iridium complex.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202307812-sup-0001-CCDC_2264592.cif2.8 MB | Supporting Information |
| anie202307812-sup-0001-CCDC_2264598.cif768.2 KB | Supporting Information |
| anie202307812-sup-0001-misc_information.pdf11.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. F. Hartwig, Acc. Chem. Res. 2012, 45, 864–873;
- 1bC. Cheng, J. F. Hartwig, Chem. Rev. 2015, 115, 8946–8975;
- 1cZ. Xu, W.-S. Huang, J. Zhang, L.-W. Xu, Synthesis 2015, 47, 3645–3668;
- 1dY.-M. Cui, Y. Lin, L.-W. Xu, Coord. Chem. Rev. 2017, 330, 37–52;
- 1eY. Fukumoto, N. Chatani, Transition-Metal-Catalyzed C−H Bond Silylation, In Organosilicon Chemistry: Novel Approaches and Reactions (Eds.: T. Hiyama, M. Oestreich), Wiley-VCH, Weinheim, 2019, pp. 171–211;
- 1fS. C. Richter, M. Oestreich, Trends Chem. 2020, 2, 13–27;
- 1gB. Li, P. H. Dixneuf, Chem. Soc. Rev. 2021, 50, 5062–5085;
- 1hM. Zhang, S. Gao, J. Tang, L. Chen, A. Liu, S. Sheng, A. Q. Zhang, Chem. Commun. 2021, 57, 8250–8263;
- 1iL. Zheng, X.-X. Nie, Y. Wu, P. Wang, Eur. J. Org. Chem. 2021, 6006–6014;
- 1jY. Ge, X. Huang, J. Ke, C. He, Chem Catal. 2022, 2, 2898–2928;
- 1kW. Yuan, C. He, Synthesis 2022, 54, 1939–1950;
- 1lB. Su, J. F. Hartwig, Angew. Chem. Int. Ed. 2022, 61, e202113343;
- 1mL. Li, W.-S. Huang, Z. Xu, L.-W. Xu, Sci. China Chem. 2023, 66, 1654–1687.
- 2
- 2aY. Kuninobu, K. Yamauchi, N. Tamura, T. Seiki, K. Takai, Angew. Chem. Int. Ed. 2013, 52, 1520–1522;
- 2bM. Murai, H. Takeshima, H. Morita, Y. Kuninobu, K. Takai, J. Org. Chem. 2015, 80, 5407–5414;
- 2cM. Murai, Y. Takeuchi, K. Yamauchi, Y. Kuninobu, K. Takai, Chem. Eur. J. 2016, 22, 6048–6058.
- 3
- 3aM. Murai, K. Matsumoto, Y. Takeuchi, K. Takai, Org. Lett. 2015, 17, 3102–3105;
- 3bT. Shibata, T. Shizuno, T. Sasaki, Chem. Commun. 2015, 51, 7802–7804;
- 3cQ.-W. Zhang, K. An, L.-C. Liu, Y. Yue, W. He, Angew. Chem. Int. Ed. 2015, 54, 6918–6921;
- 3dW.-T. Zhao, Z.-Q. Lu, H. Zheng, X.-S. Xue, D. Zhao, ACS Catal. 2018, 8, 7997–8005.
- 4
- 4aT. Lee, T. W. Wilson, R. Berg, P. Ryberg, J. F. Hartwig, J. Am. Chem. Soc. 2015, 137, 6742–6745;
- 4bT. Lee, J. F. Hartwig, Angew. Chem. Int. Ed. 2016, 55, 8723–8727;
- 4cB. Su, J. F. Hartwig, J. Am. Chem. Soc. 2017, 139, 12137–12140;
- 4dB. Su, T.-G. Zhou, X.-W. Li, X.-R. Shao, P.-L. Xu, W.-L. Wu, J. F. Hartwig, Z.-J. Shi, Angew. Chem. Int. Ed. 2017, 56, 1092–1096;
- 4eB. Su, T. Lee, J. F. Hartwig, J. Am. Chem. Soc. 2018, 140, 18032–18038.
- 5
- 5aQ.-W. Zhang, K. An, L. C. Liu, Q. Zhang, H. Guo, W. He, Angew. Chem. Int. Ed. 2017, 56, 1125–1129;
- 5bD. Mu, W. Yuan, S. Chen, N. Wang, B. Yang, L. You, B. Zu, P. Yu, C. He, J. Am. Chem. Soc. 2020, 142, 13459–13468;
- 5cB. Yang, W. Yang, Y. Guo, L. You, C. He, Angew. Chem. Int. Ed. 2020, 59, 22217–22222;
- 5dW. Yuan, L. You, W. Lin, J. Ke, Y. Li, C. He, Org. Lett. 2021, 23, 1367–1372;
- 5eS. Chen, D. Mu, P.-L. Mai, J. Ke, Y. Li, C. He, Nat. Commun. 2021, 12, 1249;
- 5fY. Guo, M.-M. Liu, X. Zhu, L. Zhu, C. He, Angew. Chem. Int. Ed. 2021, 60, 13887–13891;
- 5gW. Ma, L.-C. Liu, K. An, T. He, W. He, Angew. Chem. Int. Ed. 2021, 60, 4245–4251;
- 5hH. Zhang, D. Zhao, ACS Catal. 2021, 11, 10748–10753.
- 6
- 6aS. Chen, J. Zhu, J. Ke, Y. Li, C. He, Angew. Chem. Int. Ed. 2022, 61, e202117820;
- 6bK. An, W. Ma, L.-C. Liu, T. He, G. Guan, Q.-W. Zhang, W. He, Nat. Commun. 2022, 13, 847;
- 6cD. Mu, S. Pan, X. Wang, X. Liao, Y. Huang, J. Chen, Chem. Commun. 2022, 58, 7388–7391.
- 7
- 7aL. You, W. Yuan, C. He, Eur. J. Org. Chem. 2021, 3079–3082;
- 7bJ. Zhu, S. Chen, C. He, J. Am. Chem. Soc. 2021, 143, 5301–5307;
- 7cJ. Zhu, C. He, Synlett 2021, 32, 1575–1580;
- 7dW. Yuan, X. Zhu, Y. Xu, C. He, Angew. Chem. Int. Ed. 2022, 61, e202204912;
- 7eJ. Gao, P.-L. Mai, Y. Ge, W. Yuan, Y. Li, C. He, ACS Catal. 2022, 12, 8476–8483;
- 7fM. -M Liu, Y. Xu, C. He, J. Am. Chem. Soc. 2023, 145, 11727–11734.
- 8
- 8aJ. Y. Corey, J. Braddock-Wilking, Chem. Rev. 1999, 99, 175–292;
- 8bJ. Y. Corey, Chem. Rev. 2011, 111, 863–1071;
- 8cJ. Y. Corey, Chem. Rev. 2016, 116, 11291–11435;
- 8dF. Ye, L.-W. Xu, Synlett 2021, 32, 1281–1288.
- 9
- 9aA. Ros, B. Estepa, P. Ramirez-Lopez, E. Alvarez, R. Fernandez, J. M. Lassaletta, J. Am. Chem. Soc. 2013, 135, 15730–15733;
- 9bD.-W. Gao, Q. Gu, S.-L. You, ACS Catal. 2014, 4, 2741–2745;
- 9cJ. Zheng, S. L. You, Angew. Chem. Int. Ed. 2014, 53, 13244–13247;
- 9dJ. A. Carmona, V. Hornillos, P. Ramirez-Lopez, A. Ros, J. Iglesias-Siguenza, E. Gomez-Bengoa, R. Fernandez, J. M. Lassaletta, J. Am. Chem. Soc. 2018, 140, 11067–11075;
- 9eA. Romero-Arenas, V. Hornillos, J. Iglesias-Siguenza, R. Fernandez, J. Lopez-Serrano, A. Ros, J. M. Lassaletta, J. Am. Chem. Soc. 2020, 142, 2628–2639;
- 9fW. W. Zhang, Q. Wang, S. Z. Zhang, C. Zheng, S. L. You, Angew. Chem. Int. Ed. 2023, 62, e202214460.
- 10
- 10aP. Ducos, V. Liautard, F. Robert, Y. Landais, Chem. Eur. J. 2015, 21, 11573–11578;
- 10bV. H. G. Rohde, M. F. Müller, M. Oestreich, Organometallics 2015, 34, 3358–3373;
- 10cD. Wang, Y. Zhao, C. Yuan, J. Wen, Y. Zhao, Z. Shi, Angew. Chem. Int. Ed. 2019, 58, 12529–12533;
- 10dJ. Feng, X. Bi, X. Xue, N. Li, L. Shi, Z. Gu, Nat. Commun. 2020, 11, 4449;
- 10eX.-W. Gu, Y.-L. Sun, J.-L. Xie, X.-B. Wang, Z. Xu, G.-W. Yin, L. Li, K.-F. Yang, L.-W. Xu, Nat. Commun. 2020, 11, 2904;
- 10fX. Bi, J. Feng, X. Xue, Z. Gu, Org. Lett. 2021, 23, 3201–3206.
- 11P. Sangtrirutnugul, T. D. Tilley, Organometallics 2007, 26, 5557–5568.
- 12
- 12aT. Komuro, T. Kitano, N. Yamahira, K. Ohta, S. Okawara, N. Mager, M. Okazaki, H. Tobita, Organometallics 2016, 35, 1209–1217;
- 12bT. Kitano, T. Komuro, R. Ono, H. Tobita, Organometallics 2017, 36, 2710–2713.
- 13
- 13aJ. Takaya, N. Iwasawa, Heavier Group 14 Elements-Based Pincer Complexes in Catalytic Synthetic Transformations of Unsaturated Hydrocarbons, In Pincer and Pincer-Type Complexes: Applications in Organic Synthesis and Catalysis, First Edition (Eds.: K. Szabó, O. Wendt), Wiley-VCH, Weinheim, 2014, pp. 229–248;
- 13bF. J. Fernández-Alvarez, R. Lalrempuia, L. A. Oro, Coord. Chem. Rev. 2017, 350, 49–60;
- 13cH. Hollenhorst, R. McDonald, M. Ferguson, L. Turculet, Organometallics 2021, 40, 2768–2784.
- 14
- 14aL. Turculet, PSiP Transition-Metal Pincer Complexes: Synthesis, Bond Activation, and Catalysis, In Pincer and Pincer-Type Complexes: Applications in Organic Synthesis and Catalysis, First Edition (Eds.: K. Szabó, O. Wendt), Wiley-VCH, Weinheim, 2014, pp. 149–188;
- 14bM. Simon, F. Breher, Dalton Trans. 2017, 46, 7976–7997;
- 14cM. Tanabe, K. Osakada, Chapter 2 - Transition Metal Complexes of Silicon (Excluding Silylene Complexes), in Organosilicon Compounds (Ed.: V. Lee), Academic Press, San Diego, 2017, pp. 31–67;
- 14dE. Sola, Chapter 19 - Silicon-Based Pincers: Trans Influence and Functionality, in Pincer Compounds (Ed.: D. Morales-Morales), Elsevier, Amsterdam, 2018, pp. 401–413;
- 14eM. T. Whited, B. L. H. Taylor, Comments Inorg. Chem. 2020, 40, 217–276.
- 15B. Ghaffari, S. M. Preshlock, D. L. Plattner, R. J. Staples, P. E. Maligres, S. W. Krska, R. E. Maleczka, Jr, M. R. Smith, III, J. Am. Chem. Soc. 2014, 136, 14345–14348.
- 16
- 16aH. Fang, Y.-K. Choe, Y. Li, S. Shimada, Chem. Asian J. 2011, 6, 2512–2521;
- 16bC.-I. Lee, N. A. Hirscher, J. Zhou, N. Bhuvanesh, O. V. Ozerov, Organometallics 2015, 34, 3099–3102;
- 16cM.-U. Hung, L. P. Press, N. Bhuvanesh, O. V. Ozerov, Organometallics 2021, 40, 1004–1013;
- 16dR. Kawazu, T. Torigoe, Y. Kuninobu, Angew. Chem. Int. Ed. 2022, 61, e202202327;
- 16eT. Komuro, D. Mochizuki, H. Hashimoto, H. Tobita, Dalton Trans. 2022, 51, 9983–9987.
- 17Deposition numbers 2264598 (for 3 a) and 2264592 (for complex B) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 18
- 18aR. H. Howard, C. Alonso-Moreno, L. M. Broomfield, D. L. Hughes, J. A. Wright, M. Bochmann, Dalton Trans. 2009, 8667–8682;
- 18bP.-Y. Jiang, K.-F. Fan, S. Li, S.-H. Xiang, B. Tan, Nat. Commun. 2021, 12, 2384.
- 19
- 19aG. Choi, H. Tsurugi, K. Mashima, J. Am. Chem. Soc. 2013, 135, 13149–13161;
- 19bC. Karmel, J. F. Hartwig, J. Am. Chem. Soc. 2020, 142, 10494–10505;
- 19cZ.-B. Yan, M. Peng, Q.-L. Chen, K. Lu, Y.-Q. Tu, K.-L. Dai, F.-M. Zhang, X.-M. Zhang, Chem. Sci. 2021, 12, 9748–9753.
- 20E. M. Simmons, J. F. Hartwig, Angew. Chem. Int. Ed. 2012, 51, 3066–3072.
- 21C. Karmel, Z. Chen, J. F. Hartwig, J. Am. Chem. Soc. 2019, 141, 7063–7072.