Dynamic Control of Cyclic Peptide Assembly to Form Higher-Order Assemblies
Dr. Chongyang Wu
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Conceptualization (equal), Data curation (lead), Formal analysis (equal), Methodology (lead), Writing - original draft (equal), Writing - review & editing (equal)
Search for more papers by this authorHongyue Zhang
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Data curation (supporting), Formal analysis (supporting), Methodology (supporting), Writing - original draft (supporting)
Search for more papers by this authorNan Kong
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Data curation (supporting), Methodology (supporting)
Search for more papers by this authorBihan Wu
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Formal analysis (supporting), Methodology (supporting)
Search for more papers by this authorXinhui Lin
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Methodology (supporting)
Search for more papers by this authorCorresponding Author
Prof. Dr. Huaimin Wang
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Westlake Laboratory of Life Sciences and Biomedicine, School of Life Sciences, Westlake University, Hangzhou, 310024 Zhejiang, China
Contribution: Conceptualization (lead), Data curation (lead), Formal analysis (lead), Funding acquisition (lead), Methodology (equal), Project administration (lead), Resources (lead), Supervision (lead), Writing - original draft (lead), Writing - review & editing (lead)
Search for more papers by this authorDr. Chongyang Wu
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Conceptualization (equal), Data curation (lead), Formal analysis (equal), Methodology (lead), Writing - original draft (equal), Writing - review & editing (equal)
Search for more papers by this authorHongyue Zhang
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Data curation (supporting), Formal analysis (supporting), Methodology (supporting), Writing - original draft (supporting)
Search for more papers by this authorNan Kong
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Data curation (supporting), Methodology (supporting)
Search for more papers by this authorBihan Wu
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Formal analysis (supporting), Methodology (supporting)
Search for more papers by this authorXinhui Lin
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Contribution: Methodology (supporting)
Search for more papers by this authorCorresponding Author
Prof. Dr. Huaimin Wang
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Dunyu Road, Hangzhou, 310024 Zhejiang Province, China
Westlake Laboratory of Life Sciences and Biomedicine, School of Life Sciences, Westlake University, Hangzhou, 310024 Zhejiang, China
Contribution: Conceptualization (lead), Data curation (lead), Formal analysis (lead), Funding acquisition (lead), Methodology (equal), Project administration (lead), Resources (lead), Supervision (lead), Writing - original draft (lead), Writing - review & editing (lead)
Search for more papers by this authorGraphical Abstract
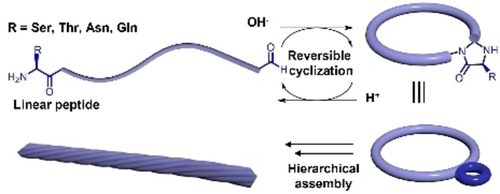
Asymmetrical cyclic peptides constructed by dynamic covalent chemistry provide a platform for the investigation of naturally occurring chirality inversion, ring-chain tautomerism, and hierarchical assembly. The insertion of a 4-imidazolidinone ring promotes the formation of intertwined nanostructures. The reversibility can be tuned by changing the pH value without producing other chemical waste except water.
Abstract
Chirality correction, asymmetry, ring-chain tautomerism and hierarchical assemblies are fundamental phenomena in nature. They are geometrically related and may impact the biological roles of a protein or other supermolecules. It is challenging to study those behaviors within an artificial system due to the complexity of displaying these features. Herein, we design an alternating D,L peptide to recreate and validate the naturally occurring chirality inversion prior to cyclization in water. The resulting asymmetrical cyclic peptide containing a 4-imidazolidinone ring provides an excellent platform to study the ring-chain tautomerism, thermostability and dynamic assembly of the nanostructures. Different from traditional cyclic D,L peptides, the formation of 4-imidazolidinone promotes the formation of intertwined nanostructures. Analysis of the nanostructures confirmed the left-handedness, representing chirality induced self-assembly. This proves that a rationally designed peptide can mimic multiple natural phenomena and could promote the development of functional biomaterials, catalysts, antibiotics, and supermolecules.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202303455-sup-0001-misc_information.pdf5.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Rustom, R. Saffrich, I. Markovic, P. Walther, H.-H. Gerdes, Science 2004, 303, 1007–1010;
- 1bD. M. Davis, S. Sowinski, Nat. Rev. Mol. Cell Biol. 2008, 9, 431–436.
- 2Introduction to Biotechnology: the Science, Technology and Medical Applications (Ed.: W. T. Godbey), Woodhead Publishing, London, 2014.
- 3
- 3aJ. T. Davis, O. Okunola, R. Quesada, Chem. Soc. Rev. 2010, 39, 3843–3862;
- 3bJ. R. Granja, M. R. Ghadiri, J. Am. Chem. Soc. 1994, 116, 10785–10786.
- 4Y. Lin, S. Taylor, H. Li, K. A. S. Fernando, L. Qu, W. Wang, L. Gu, B. Zhou, Y.-P. Sun, J. Mater. Chem. 2004, 14, 527–541.
- 5R. Duncan, R. Gaspar, Mol. Pharmaceutics 2011, 8, 2101–2141.
- 6J. Chen, B. Zhang, F. Xia, Y. Xie, S. Jiang, R. Su, Y. Lu, W. Wu, Nanoscale 2016, 8, 7127–7136.
- 7
- 7aX. Xie, T. Zheng, W. Li, Macromol. Rapid Commun. 2020, 41, 2000534;
- 7bJ. Wang, L. Hu, H. Zhang, Y. Fang, T. Wang, H. Wang, Adv. Mater. 2022, 34, 2104704;
- 7cH. Wang, Z. Feng, B. Xu, Angew. Chem. Int. Ed. 2019, 131, 10532–10541.
- 8
- 8aD.-S. Yang, Y.-H. Yang, Y. Zhou, L.-L. Yu, R.-H. Wang, B. Di, M.-M. Niu, Adv. Funct. Mater. 2020, 30, 1904969;
- 8bA. S. Carlini, R. Gaetani, R. L. Braden, C. Luo, K. L. Christman, N. C. Gianneschi, Nat. Commun. 2019, 10, 1735.
- 9
- 9aK. Hu, W. Xiong, C. Sun, C. Wang, J. Li, F. Yin, Y. Jiang, M.-R. Zhang, Z. Li, X. Wang, Z. Li, CCS Chem. 2020, 2, 42–51;
- 9bA. Fuertes, M. Juanes, J. R. Granja, J. Montenegro, Chem. Commun. 2017, 53, 7861–7871;
- 9cT. Shimizu, W. Ding, N. Kameta, Chem. Rev. 2020, 120, 2347–2407;
- 9dN. Rodríguez-Vázquez, R. García-Fandiño, M. Amorín, J. R. Granja, Chem. Sci. 2016, 7, 183–187;
- 9eJ. M. Priegue, I. Louzao, I. Gallego, J. Montenegro, J. R. Granja, Org. Chem. Front. 2022, 9, 1226–1233;
- 9fR. Chapman, M. Danial, M. L. Koh, K. A. Jolliffe, S. Perrier, Chem. Soc. Rev. 2012, 41, 6023–6041;
- 9gQ. Song, J. Yang, J. Y. Rho, S. Perrier, Chem. Commun. 2019, 55, 5291–5294;
- 9hQ. Song, Z. Cheng, M. Kariuki, S. C. L. Hall, S. K. Hill, J. Y. Rho, S. Perrier, Chem. Rev. 2021, 121, 13936–13995.
- 10M. R. Ghadiri, J. R. Granja, R. A. Milligan, D. E. McRee, N. Khazanovich, Nature 1993, 366, 324–327.
- 11
- 11aC. Yuan, W. Ji, R. Xing, J. Li, E. Gazit, X. Yan, Nat. Chem. Rev. 2019, 3, 567–588;
- 11bJ. Yang, X. Yu, J.-I. Song, Q. Song, S. C. L. Hall, G. Yu, S. Perrier, Angew. Chem. Int. Ed. 2022, 61, e202115208;
- 11cJ. Y. Rho, H. Cox, E. D. H. Mansfield, S. H. Ellacott, R. Peltier, J. C. Brendel, M. Hartlieb, T. A. Waigh, S. Perrier, Nat. Commun. 2019, 10, 4708;
- 11dS. C. Larnaudie, J. C. Brendel, I. Romero-Canelón, C. Sanchez-Cano, S. Catrouillet, J. Sanchis, J. P. C. Coverdale, J.-I. Song, A. Habtemariam, P. J. Sadler, K. A. Jolliffe, S. Perrier, Biomacromolecules 2018, 19, 239–247.
- 12J. E. Becker, R. E. Moore, B. S. Moore, Gene 2004, 325, 35–42.
- 13T. Jiao, G. Wu, Y. Zhang, L. Shen, Y. Lei, C.-Y. Wang, A. C. Fahrenbach, H. Li, Angew. Chem. Int. Ed. 2020, 59, 18350–18367.
- 14L. R. Malins, J. N. deGruyter, K. J. Robbins, P. M. Scola, M. D. Eastgate, M. R. Ghadiri, P. S. Baran, J. Am. Chem. Soc. 2017, 139, 5233–5241.
- 15
- 15aV. Adebomi, R. D. Cohen, R. Wills, H. A. H. Chavers, G. E. Martin, M. Raj, Angew. Chem. Int. Ed. 2019, 58, 19073–19080;
- 15bR. Wills, V. Adebomi, C. Spancake, R. D. Cohen, M. Raj, Tetrahedron 2022, 126, 133071.
- 16
- 16aN. Rodríguez-Vázquez, M. Amorín, I. Alfonso, J. R. Granja, Angew. Chem. Int. Ed. 2016, 55, 4504–4508;
- 16bJ. Montenegro, M. R. Ghadiri, J. R. Granja, Acc. Chem. Res. 2013, 46, 2955–2965;
- 16cR. J. Brea, C. Reiriz, J. R. Granja, Chem. Soc. Rev. 2010, 39, 1448–1456.
- 17
- 17aI. Insua, J. Montenegro, J. Am. Chem. Soc. 2020, 142, 300–307;
- 17bS. Díaz, I. Insua, G. Bhak, J. Montenegro, Chem. Eur. J. 2020, 26, 14765–14770.
- 18A. Méndez-Ardoy, J. R. Granja, J. Montenegro, Nanoscale Horiz. 2018, 3, 391–396.
- 19
- 19aT. Golakoti, W. Y. Yoshida, S. Chaganty, R. E. Moore, J. Nat. Prod. 2001, 64, 54–59;
- 19bJ. Jokela, L. Herfindal, M. Wahlsten, P. Permi, F. Selheim, V. Vasconçelos, S. O. Døskeland, K. Sivonen, ChemBioChem 2010, 11, 1594–1599.
- 20
- 20aS. Enck, F. Kopp, M. A. Marahiel, A. Geyer, ChemBioChem 2008, 9, 2597–2601;
- 20bS. Enck, F. Kopp, M. A. Marahiel, A. Geyer, Org. Biomol. Chem. 2010, 8, 559–563.
- 21
- 21aJ. V. Paukstelis, L. L. Lambing, Tetrahedron Lett. 1970, 11, 299–302;
10.1016/S0040-4039(00)61813-0 Google Scholar
- 21bF. Fulop, K. Pihlaja, K. Neuvonen, G. Bernath, G. Argay, A. Kalman, J. Org. Chem. 1993, 58, 1967–1969;
- 21cL. Lázár, F. Fülöp, Eur. J. Org. Chem. 2003, 3025–3042.
- 22D. Bermejo-Velasco, G. N. Nawale, O. P. Oommen, J. Hilborn, O. P. Varghese, Chem. Commun. 2018, 54, 12507–12510.
- 23S. W. Larsen, M. Sidenius, M. Ankersen, C. Larsen, Eur. J. Pharm. Sci. 2003, 20, 233–240.
- 24
- 24aS. I. Al-Gharabli, S. T. A. Shah, S. Weik, M. F. Schmidt, J. R. Mesters, D. Kuhn, G. Klebe, R. Hilgenfeld, J. Rademann, ChemBioChem 2006, 7, 1048–1055;
- 24bY. Wu, C. Li, S. Fan, Y. Zhao, C. Wu, Bioconjugate Chem. 2021, 32, 2065–2072.
- 25
- 25aH. N. Hoang, C. Y. Wu, T. A. Hill, A. D. de Araujo, P. V. Bernhardt, L. G. Liu, D. P. Fairlie, Angew. Chem. Int. Ed. 2019, 58, 18873–18877;
- 25bR. W. Newberry, R. T. Raines, Acc. Chem. Res. 2017, 50, 1838–1846.
- 26M. Ferstl, A. Strasser, H.-J. Wittmann, M. Drechsler, M. Rischer, J. Engel, A. Goepferich, Langmuir 2011, 27, 14450–14459.
- 27K. Koner, S. Karak, S. Kandambeth, S. Karak, N. Thomas, L. Leanza, C. Perego, L. Pesce, R. Capelli, M. Moun, M. Bhakar, T. G. Ajithkumar, G. M. Pavan, R. Banerjee, Nat. Chem. 2022, 14, 507–514.
- 28
- 28aV. Castelletto, J. Seitsonen, J. Ruokolainen, I. W. Hamley, Soft Matter 2021, 17, 3096–3104;
- 28bB. Stuart, Biological Applications of Infrared Spectroscopy, Wiley, New York, 1997;
- 28cI. W. Hamley, Introduction to Peptide Science, Wiley & Sons, Inc., Hoboken, 2020.
- 29
- 29aS.-J. Choi, W.-J. Jeong, S.-K. Kang, M. Lee, E. Kim, D. Y. Ryu, Y.-B. Lim, Biomacromolecules 2012, 13, 1991–1995;
- 29bC. E. Dempsey, P. E. Mason, P. Jungwirth, J. Am. Chem. Soc. 2011, 133, 7300–7303.
- 30
- 30aF. Wang, O. Gnewou, S. Wang, T. Osinski, X. Zuo, E. H. Egelman, V. P. Conticello, Matter 2021, 4, 3217–3231;
- 30bL. Pieri, F. Wang, A.-A. Arteni, M. Vos, J.-M. Winter, M.-H. Le Du, F. Artzner, F. Gobeaux, P. Legrand, Y. Boulard, S. Bressanelli, E. H. Egelman, M. Paternostre, Proc. Natl. Acad. Sci. USA 2022, 119, e2120346119.