Mechanistic Elucidations of Highly Dispersed Metalloporphyrin Metal-Organic Framework Catalysts for CO2 Electroreduction
Michael R. Smith
Department of Chemistry, Princeton University, Princeton, NJ 08544 USA
These authors contributed equally to this work.
Search for more papers by this authorClare B. Martin
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
These authors contributed equally to this work.
Search for more papers by this authorSonia Arumuganainar
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
Search for more papers by this authorAri Gilman
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
Search for more papers by this authorBruce E. Koel
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
Search for more papers by this authorCorresponding Author
Michele L. Sarazen
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
Search for more papers by this authorMichael R. Smith
Department of Chemistry, Princeton University, Princeton, NJ 08544 USA
These authors contributed equally to this work.
Search for more papers by this authorClare B. Martin
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
These authors contributed equally to this work.
Search for more papers by this authorSonia Arumuganainar
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
Search for more papers by this authorAri Gilman
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
Search for more papers by this authorBruce E. Koel
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
Search for more papers by this authorCorresponding Author
Michele L. Sarazen
Department of Chemical and Biological Engineering, Princeton University, Princeton, NJ 08544 USA
Search for more papers by this authorGraphical Abstract
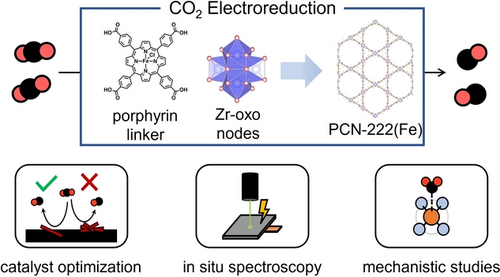
PCN-222(Fe), a metalloporphyrin-based metal–organic framework (MOF), was used for the CO2 electroreduction reaction. The proposed reaction mechanism necessitated catalyst weight loadings of 1 wt.% to mitigate obfuscations from mass transfer and in situ Raman spectroscopy confirmed the structural and reactive stability of the MOF under operating conditions.
Abstract
Immobilization of porphyrin complexes into crystalline metal–organic frameworks (MOFs) enables high exposure of porphyrin active sites for CO2 electroreduction. Herein, well-dispersed iron-porphyrin-based MOF (PCN-222(Fe)) on carbon-based electrodes revealed optimal turnover frequencies for CO2 electroreduction to CO at 1 wt.% catalyst loading, beyond which the intrinsic catalyst activity declined due to CO2 mass transport limitations. In situ Raman suggested that PCN-222(Fe) maintained its structure under electrochemical bias, permitting mechanistic investigations. These revealed a stepwise electron transfer-proton transfer mechanism for CO2 electroreduction on PCN-222(Fe) electrodes, which followed a shift from a rate-limiting electron transfer to CO2 mass transfer as the potential increased from −0.6 V to −1.0 V vs. RHE. Our results demonstrate how intrinsic catalytic investigations and in situ spectroscopy are needed to elucidate CO2 electroreduction mechanisms on PCN-222(Fe) MOFs.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202218208-sup-0001-misc_information.pdf11.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo, E. H. Sargent, Science 2019, 364, 350.
- 2H. Jahangiri, J. Bennett, P. Mahjoubi, K. Wilson, S. Gu, Catal. Sci. Technol. 2014, 4, 2210–2229.
- 3R. G. Grim, A. T. To, C. A. Farberow, J. E. Hensley, D. A. Ruddy, J. A. Schaidle, ACS Catal. 2019, 9, 4145–4172.
- 4Y. Hori, in Mod. Asp. Electrochem., Springer, New York, 2008, p. 89. https://doi.org/10.1007/978-0-387-49489-0 3.
10.1007/978-0-387-49489-0_3 Google Scholar
- 5R. Francke, B. Schille, M. Roemelt, Chem. Rev. 2018, 118, 4631–4701.
- 6A. R. Paris, A. B. Bocarsly, ACS Catal. 2019, 9, 2324–2333.
- 7P. De Luna, R. Quintero-Bermudez, C. T. Dinh, M. B. Ross, O. S. Bushuyev, P. Todorović, T. Regier, S. O. Kelley, P. Yang, E. H. Sargent, Nat. Catal. 2018, 1, 103–110.
- 8X. Zheng, P. De Luna, F. P. García de Arquer, B. Zhang, N. Becknell, M. B. Ross, Y. Li, M. N. Banis, Y. Li, M. Liu, O. Voznyy, C. T. Dinh, T. Zhuang, P. Stadler, Y. Cui, X. Du, P. Yang, E. H. Sargent, Joule 2017, 1, 794–805.
- 9A. R. Paris, A. T. Chu, C. B. O'Brien, J. J. Frick, S. A. Francis, A. B. Bocarsly, J. Electrochem. Soc. 2018, 165, H385–H392.
- 10K. Y. Cohen, R. Evans, S. Dulovic, A. B. Bocarsly, Acc. Chem. Res. 2022, 55, 944–954.
- 11Y. Wu, J. Jiang, Z. Weng, M. Wang, D. L. J. Broere, Y. Zhong, G. W. Brudvig, Z. Feng, H. Wang, ACS Cent. Sci. 2017, 3, 847–852.
- 12Z. Weng, J. Jiang, Y. Wu, Z. Wu, X. Guo, K. L. Materna, W. Liu, V. S. Batista, G. W. Brudvig, H. Wang, J. Am. Chem. Soc. 2016, 138, 8076–8079.
- 13M. Hammouche, D. Lexa, J. M. Savéant, M. Momenteau, J. Electroanal. Chem. Interfacial Electrochem. 1988, 249, 347–351.
- 14N. Morlanés, K. Takanabe, V. Rodionov, ACS Catal. 2016, 6, 3092–3095.
- 15H. Noh, C. W. Kung, K. I. Otake, A. W. Peters, Z. Li, Y. Liao, X. Gong, O. K. Farha, J. T. Hupp, ACS Catal. 2018, 8, 9848–9858.
- 16S. Amanullah, P. Saha, R. Saha, A. Dey, Inorg. Chem. 2019, 58, 152–164.
- 17I. Hod, M. D. Sampson, P. Deria, C. P. Kubiak, O. K. Farha, J. T. Hupp, ACS Catal. 2015, 5, 6302–6309.
- 18M. Abdinejad, K. Tang, C. Dao, S. Saedy, T. Burdyny, J. Mater. Chem. A 2022, 10, 7626–7636.
- 19A. Maurin, M. Robert, Chem. Commun. 2016, 52, 12084–12087.
- 20A. Maurin, M. Robert, J. Am. Chem. Soc. 2016, 138, 2492–2495.
- 21M. Zhu, R. Ye, K. Jin, N. Lazouski, K. Manthiram, ACS Energy Lett. 2018, 3, 1381–1386.
- 22D. Grammatico, A. J. Bagnall, L. Riccardi, M. Fontecave, B.-L. Su, L. Billon, Angew. Chem. Int. Ed. 2022, 61, e202206399; Angew. Chem. 2022, 134, e202206399.
- 23L. Jiao, W. Yang, G. Wan, R. Zhang, X. Zheng, H. Zhou, S. H. Yu, H. L. Jiang, Angew. Chem. Int. Ed. 2020, 59, 20589–20595; Angew. Chem. 2020, 132, 20770–20776.
- 24B. X. Dong, S. L. Qian, F. Y. Bu, Y. C. Wu, L. G. Feng, Y. L. Teng, W. L. Liu, Z. W. Li, ACS Appl. Energy Mater. 2018, 1, 4662–4669.
- 25N. Kornienko, Y. Zhao, C. S. Kley, C. Zhu, D. Kim, S. Lin, C. J. Chang, O. M. Yaghi, P. Yang, J. Am. Chem. Soc. 2015, 137, 14129–14135.
- 26S. Lin, C. S. Diercks, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi, C. J. Chang, Science 2015, 349, 1208–1213.
- 27X. Xie, X. Zhang, M. Xie, L. Xiong, H. Sun, Y. Lu, Q. Mu, M. H. Rummeli, J. Xu, S. Li, J. Zhong, Z. Deng, B. Ma, T. Cheng, W. A. Goddard, Y. Peng, Nat. Commun. 2022, 13, 63.
- 28X. Yang, Q.-X. Li, S.-Y. Chi, H.-F. Li, Y.-B. Huang, R. Cao, C. Hong-Fang Li, SmartMat 2022, 3, 163–172.
- 29B. A. Johnson, A. M. Beiler, B. D. McCarthy, S. Ott, J. Am. Chem. Soc. 2020, 142, 11941–11956.
- 30Y. Peng, S. Sanati, A. Morsali, H. García, Angew. Chem. Int. Ed. 2023, 62, e202214707; Angew. Chem. 2023, 135, e202214707.
- 31D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei, H.-C. Zhou, Angew. Chem. Int. Ed. 2012, 51, 10307–10310; Angew. Chem. 2012, 124, 10453–10456.
- 32W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart, O. M. Yaghi, Inorg. Chem. 2012, 51, 6443–6445.
- 33B. Chen, T. Jiang, H. Fu, X. Qu, Z. Xu, S. Zheng, Anal. Chim. Acta 2021, 1145, 95–102.
- 34P. Kucheryavy, N. Lahanas, E. Velasco, C. J. Sun, J. V. Lockard, J. Phys. Chem. Lett. 2016, 7, 1109–1115.
- 35P. Kucheryavy, N. Lahanas, J. V. Lockard, Inorg. Chem. 2018, 57, 3339–3347.
- 36M. Aghayan, A. Mahmoudi, S. Sohrabi, S. Dehghanpour, K. Nazari, N. Mohammadian-Tabrizi, Anal. Methods 2019, 11, 3175–3187.
- 37K. Liu, W. A. Smith, T. Burdyny, ACS Energy Lett. 2019, 4, 639–643.
- 38Z. Xing, L. Hu, D. S. Ripatti, X. Hu, X. Feng, Nat. Commun. 2021, 12, 136.
- 39R. G. Mariano, O. J. Wahab, J. A. Rabinowitz, J. Oppenheim, T. Chen, P. R. Unwin, M. Dincǎ, ACS Cent. Sci. 2022, 8, 975–982.
- 40W. W. Kramer, C. C. L. McCrory, Chem. Sci. 2016, 7, 2506–2515.
- 41L. W. Xu, S. L. Qian, B. X. Dong, L. G. Feng, Z. W. Li, J. Mater. Sci. 2022, 57, 526–537.
- 42J. Choi, J. Kim, P. Wagner, S. Gambhir, R. Jalili, S. Byun, S. Sayyar, Y. M. Lee, D. R. MacFarlane, G. G. Wallace, D. L. Officer, Energy Environ. Sci. 2019, 12, 747–755.
- 43S. Aoi, K. Mase, K. Ohkubo, S. Fukuzumi, Chem. Commun. 2015, 51, 10226–10228.
- 44X. Zhang, Z. Wu, X. Zhang, L. Li, Y. Li, H. Xu, X. Li, X. Yu, Z. Zhang, Y. Liang, H. Wang, Nat. Commun. 2017, 8, 14675.
- 45K. Yang, R. Kas, W. A. Smith, T. Burdyny, ACS Energy Lett. 2021, 6, 33–40.
- 46I. Azcarate, C. Costentin, M. Robert, J.-M. Saveánt, J. Phys. Chem. C 2016, 120, 28951–28960.
- 47I. Azcarate, C. Costentin, M. Robert, J.-M. Saveánt, J. Am. Chem. Soc. 2016, 138, 16639–16644.
- 48C. T. Dinh, T. Burdyny, G. Kibria, A. Seifitokaldani, C. M. Gabardo, F. Pelayo García De Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Zou, R. Quintero-Bermudez, Y. Pang, D. Sinton, E. H. Sargent, Science 2018, 360, 783–787.
- 49Z. Weng, Y. Wu, M. Wang, J. Jiang, K. Yang, S. Huo, X. F. Wang, Q. Ma, G. W. Brudvig, V. S. Batista, Y. Liang, Z. Feng, H. Wang, Nat. Commun. 2018, 9, 415.
- 50W. Zheng, L. Y. S. Lee, ACS Energy Lett. 2021, 6, 2838–2843.
- 51M. R. Smith, A. Gilman, C. W. Hullfish, W. Niu, Y. Zheng, B. E. Koel, M. L. Sarazen, J. Phys. Chem. C 2022, 126, 13649–13659.
- 52P. Lasch, Chemom. Intell. Lab. Syst. 2012, 117, 100–114.
- 53D.-H. Nam, O. Shekhah, G. Lee, A. Mallick, H. Jiang, F. Li, B. Chen, J. Wicks, M. Eddaoudi, E. H. Sargent, J. Am. Chem. Soc. 2020, 142, 21513–21521.
- 54R. Shimoni, Z. Shi, S. Binyamin, Y. Yang, I. Liberman, R. Ifraemov, S. Mukhopadhyay, L. Zhang, I. Hod, Angew. Chem. Int. Ed. 2022, 61, e202206085; Angew. Chem. 2022, 134, e202206085.
- 55A. Wuttig, M. Yaguchi, K. Motobayashi, M. Osawa, Y. Surendranath, Proc. Natl. Acad. Sci. USA 2016, 113, E4585–E4593.
- 56M. E. Leonard, L. E. Clarke, A. Forner-Cuenca, S. M. Brown, F. R. Brushett, ChemSusChem 2020, 13, 400–411.
- 57S. Lin, P. M. Usov, A. J. Morris, Chem. Commun. 2018, 54, 6965.
- 58S. R. Ahrenholtz, C. C. Epley, A. J. Morris, J. Am. Chem. Soc. 2014, 136, 2464–2472.