Tuning the CO2 Hydrogenation Selectivity of Rhodium Single-Atom Catalysts on Zirconium Dioxide with Alkali Ions
Shang Li
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorYuxing Xu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Dr. Hengwei Wang
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Botao Teng
Tianjin Key Laboratory of Brine Chemical Engineering and Resource Eco-utilization, College of Chemical Engineering and Materials Science, Tianjin University of Science and Technology, Tianjin, 300457 China
Search for more papers by this authorDr. Qin Liu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Qiuhua Li
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorLulu Xu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorXinyu Liu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Junling Lu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorShang Li
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorYuxing Xu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Dr. Hengwei Wang
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Botao Teng
Tianjin Key Laboratory of Brine Chemical Engineering and Resource Eco-utilization, College of Chemical Engineering and Materials Science, Tianjin University of Science and Technology, Tianjin, 300457 China
Search for more papers by this authorDr. Qin Liu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Qiuhua Li
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorLulu Xu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorXinyu Liu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Junling Lu
Department of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorGraphical Abstract
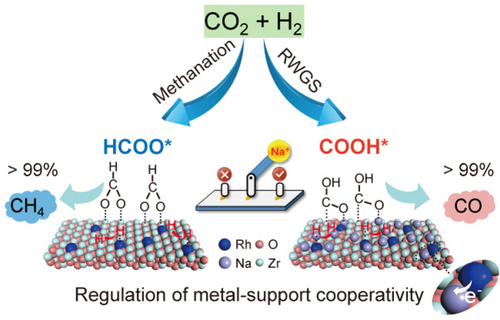
Doping alkali ions (e.g., Na) into Rh1/ZrO2 enables complete switching of the reaction product from CH4 to CO in CO2 hydrogenation over a broad temperature range. It was found that Na+ ions change the surface intermediate from HCOO* to COOH* and render the Rh1 atoms electron deficient with restrained H2 activation capability and weakened CO adsorption, thus cooperatively boosting CO formation.
Abstract
Tuning the coordination environments of metal single atoms (M1) in single-atom catalysts has shown large impacts on catalytic activity and stability but often barely on selectivity in thermocatalysis. Here, we report that simultaneously regulating both Rh1 atoms and ZrO2 support with alkali ions (e.g., Na) enables efficient switching of the reaction products from nearly 100 % CH4 to above 99 % CO in CO2 hydrogenation in a wide temperature range (240–440 °C) along with a record high activity of 9.4 molCO gRh−1 h−1 at 300 °C and long-term stability. In situ spectroscopic characterization and theoretical calculations unveil that alkali ions on ZrO2 change the surface intermediate from formate to carboxy species during CO2 activation, thus leading to exclusive CO formation. Meanwhile, alkali ions also reinforce the electronic Rh1-support interactions, endowing the Rh1 atoms more electron deficient, which improves the stability against sintering and inhibits deep hydrogenation of CO to CH4.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202218167-sup-0001-misc_information.pdf3.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Wang, J. Li, T. Zhang, Nat. Chem. Rev. 2018, 2, 65–81;
- 1bS. K. Kaiser, Z. Chen, D. Faust Akl, S. Mitchell, J. Perez-Ramirez, Chem. Rev. 2020, 120, 11703–11809.
- 2
- 2aL. Liu, A. Corma, Chem. Rev. 2018, 118, 4981–5079;
- 2bB. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li, T. Zhang, Nat. Chem. 2011, 3, 634–641.
- 3
- 3aL. Zhang, M. Zhou, A. Wang, T. Zhang, Chem. Rev. 2020, 120, 683–733;
- 3bP. Liu, Y. Zhao, R. Qin, S. Mo, G. Chen, L. Gu, M. Chevrier Daniel, P. Zhang, Q. Guo, D. Zang, B. Wu, G. Fu, N. Zheng, Science 2016, 352, 797–800;
- 3cL. Wang, C. Zhu, M. Xu, C. Zhao, J. Gu, L. Cao, X. Zhang, Z. Sun, S. Wei, W. Zhou, W. X. Li, J. Lu, J. Am. Chem. Soc. 2021, 143, 18854–18858.
- 4X. F. Yang, A. Q. Wang, B. T. Qiao, J. Li, J. Y. Liu, T. Zhang, Acc. Chem. Res. 2013, 46, 1740–1748.
- 5
- 5aJ. Li, Q. Guan, H. Wu, W. Liu, Y. Lin, Z. Sun, X. Ye, X. Zheng, H. Pan, J. Zhu, S. Chen, W. Zhang, S. Wei, J. Lu, J. Am. Chem. Soc. 2019, 141, 14515–14519;
- 5bY. Ren, Y. Tang, L. Zhang, X. Liu, L. Li, S. Miao, D. Sheng Su, A. Wang, J. Li, T. Zhang, Nat. Commun. 2019, 10, 4500;
- 5cH. Jeong, D. Shin, B. S. Kim, J. Bae, S. Shin, C. Choe, J. W. Han, H. Lee, Angew. Chem. Int. Ed. 2020, 59, 20691–20696; Angew. Chem. 2020, 132, 20872–20877;
- 5dD. Huang, N. He, Q. Zhu, C. Chu, S. Weon, K. Rigby, X. Zhou, L. Xu, J. Niu, E. Stavitski, J.-H. Kim, ACS Catal. 2021, 11, 5586–5592.
- 6
- 6aY. Pan, Y. Chen, K. Wu, Z. Chen, S. Liu, X. Cao, W. C. Cheong, T. Meng, J. Luo, L. Zheng, C. Liu, D. Wang, Q. Peng, J. Li, C. Chen, Nat. Commun. 2019, 10, 4290;
- 6bZ. Chen, E. Vorobyeva, S. Mitchell, E. Fako, N. López, S. M. Collins, R. K. Leary, P. A. Midgley, R. Hauert, J. Pérez-Ramírez, Natl. Sci. Rev. 2018, 5, 642–652;
- 6cL. Jiang, K. Liu, S.-F. Hung, L. Zhou, R. Qin, Q. Zhang, P. Liu, L. Gu, H. M. Chen, G. Fu, N. Zheng, Nat. Nanotechnol. 2020, 15, 848–853.
- 7G. X. Pei, X. Y. Liu, X. Yang, L. Zhang, A. Wang, L. Li, H. Wang, X. Wang, T. Zhang, ACS Catal. 2017, 7, 1491–1500.
- 8J. Yang, W. Li, D. Wang, Y. Li, Adv. Mater. 2020, 32, 2003300.
- 9
- 9aJ. Zhang, M. Wang, Z. Gao, X. Qin, Y. Xu, Z. Wang, W. Zhou, D. Ma, J. Am. Chem. Soc. 2022, 144, 5108–5115;
- 9bH. Li, L. Wang, Y. Dai, Z. Pu, Z. Lao, Y. Chen, M. Wang, X. Zheng, J. Zhu, W. Zhang, R. Si, C. Ma, J. Zeng, Nat. Nanotechnol. 2018, 13, 411–417.
- 10
- 10aM. Gao, J. Zhang, P. Zhu, X. Liu, Z. Zheng, Appl. Catal. B 2022, 314, 121476;
- 10bP. Panagiotopoulou, Appl. Catal. B 2018, 236, 162–170;
- 10cJ. M. Pigos, C. J. Brooks, G. Jacobs, B. H. Davis, Appl. Catal. A 2007, 328, 14–26;
- 10dZ. Shi, H. Yang, P. Gao, X. Chen, H. Liu, L. Zhong, H. Wang, W. Wei, Y. Sun, Chin. J. Catal. 2018, 39, 1294–1302.
- 11J. Qi, P. Christopher, Ind. Eng. Chem. Res. 2019, 58, 12632–12641.
- 12Y. Kwon, T. Y. Kim, G. Kwon, J. Yi, H. Lee, J. Am. Chem. Soc. 2017, 139, 17694–17699.
- 13J. C. Matsubu, V. N. Yang, P. Christopher, J. Am. Chem. Soc. 2015, 137, 3076–3084.
- 14R. Lang, T. Li, D. Matsumura, S. Miao, Y. Ren, Y. T. Cui, Y. Tan, B. Qiao, L. Li, A. Wang, X. Wang, T. Zhang, Angew. Chem. Int. Ed. 2016, 55, 16054–16058; Angew. Chem. 2016, 128, 16288–16292.
- 15O. Martin, A. J. Martin, C. Mondelli, S. Mitchell, T. F. Segawa, R. Hauert, C. Drouilly, D. Curulla-Ferre, J. Perez-Ramirez, Angew. Chem. Int. Ed. 2016, 55, 6261–6265; Angew. Chem. 2016, 128, 6369–6373.
- 16
- 16aH. Li, T. Chen, G. Wang, Appl. Catal. A 2022, 639, 118607;
- 16bL. F. Liotta, G. Deganello, P. Delichere, C. Leclercq, G. A. Martin, J. Catal. 1996, 164, 334–340;
- 16cC. Wang, E. Guan, L. Wang, X. Chu, Z. Wu, J. Zhang, Z. Yang, Y. Jiang, L. Zhang, X. Meng, B. C. Gates, F. S. Xiao, J. Am. Chem. Soc. 2019, 141, 8482–8488;
- 16dR. Qin, L. Zhou, P. Liu, Y. Gong, K. Liu, C. Xu, Y. Zhao, L. Gu, G. Fu, N. Zheng, Nat. Catal. 2020, 3, 703–709.
- 17
- 17aM. A. A. Aziz, A. A. Jalil, S. Triwahyono, A. Ahmad, Green Chem. 2015, 17, 2647–2663;
- 17bW. Wang, S. Wang, X. Ma, J. Gong, Chem. Soc. Rev. 2011, 40, 3703–3727.
- 18
- 18aH. Xin, L. Lin, R. Li, D. Li, T. Song, R. Mu, Q. Fu, X. Bao, J. Am. Chem. Soc. 2022, 144, 4874–4882;
- 18bX. Li, J. Lin, L. Li, Y. Huang, X. Pan, S. E. Collins, Y. Ren, Y. Su, L. Kang, X. Liu, Y. Zhou, H. Wang, A. Wang, B. Qiao, X. Wang, T. Zhang, Angew. Chem. Int. Ed. 2020, 59, 19983–19989; Angew. Chem. 2020, 132, 20158–20164.
- 19Y. Zhu, S. F. Yuk, J. Zheng, M. T. Nguyen, M. S. Lee, J. Szanyi, L. Kovarik, Z. Zhu, M. Balasubramanian, V. A. Glezakou, J. L. Fulton, J. A. Lercher, R. Rousseau, O. Y. Gutierrez, J. Am. Chem. Soc. 2021, 143, 5540–5549.
- 20E. Cremer, Adv. Catal. 1955, 7, 75–91.
- 21Y. Guo, S. Mei, K. Yuan, D.-J. Wang, H.-C. Liu, C.-H. Yan, Y.-W. Zhang, ACS Catal. 2018, 8, 6203–6215.
- 22L. Wang, W. Fang, L. Wang, F.-S. Xiao, ChemSusChem 2020, 13, 6300–6306.
- 23F. Wang, S. He, H. Chen, B. Wang, L. Zheng, M. Wei, D. G. Evans, X. Duan, J. Am. Chem. Soc. 2016, 138, 6298–6305.
- 24
- 24aX. Nie, W. Luo, M. J. Janik, A. Asthagiri, J. Catal. 2014, 312, 108–122;
- 24bY. Chen, H. Li, W. Zhao, W. Zhang, J. Li, W. Li, X. Zheng, W. Yan, W. Zhang, J. Zhu, R. Si, J. Zeng, Nat. Commun. 2019, 10, 1885.
- 25X. Wang, P. J. Ramirez, W. Liao, J. A. Rodriguez, P. Liu, J. Am. Chem. Soc. 2021, 143, 13103–13112.