Helical β-isoindigo-Based Chromophores with B−O−B Bridge: Facile Synthesis and Tunable Near-Infrared Circularly Polarized Luminescence
Yongqiang Xu
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Zhigang Ni
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
These authors contributed equally to this work.
Search for more papers by this authorYao Xiao
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorZiwei Chen
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorDr. Sisi Wang
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorLizhi Gai
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorProf. Dr. You-Xuan Zheng
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorProf. Dr. Zhen Shen
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hua Lu
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorProf. Dr. Zijian Guo
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorYongqiang Xu
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Zhigang Ni
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
These authors contributed equally to this work.
Search for more papers by this authorYao Xiao
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorZiwei Chen
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorDr. Sisi Wang
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorLizhi Gai
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorProf. Dr. You-Xuan Zheng
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorProf. Dr. Zhen Shen
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hua Lu
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, and Key Laboratory of Organosilicon Material Technology of Zhejiang Province, Hangzhou Normal University, Hangzhou, 311121 China
Search for more papers by this authorProf. Dr. Zijian Guo
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorGraphical Abstract
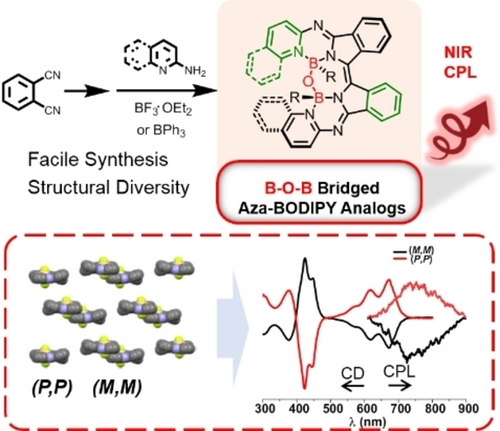
A study of a series of helical β-isoindigo-based B−O−B-bridged chromophores has shown that the bridge leads to distorted conformations. This results in the chromophores having excellent spectroscopic and chiroptical properties, such as tunable circularly polarized luminescence (CPL), with a high luminescence dissymmetry factor (glum) of 1.3×10−3 and a CPL brightness (BCPL) of 11.5 M−1 cm−1 in the near-infrared region observed for one of the compounds.
Abstract
It is essential to create organic compounds that exhibit circularly polarized luminescence (CPL) in the near-infrared (NIR) range. Helicene-type emitters possess appealing chiroptical features, however, such NIR molecules are scarce due to a paucity of synthetic strategies. Herein, we developed a series of helical β-isoindigo-based B−O−B bridged aza-BODIPY analogs that were synthesized conveniently. The reaction of diimino-β-isoindigo with a heteroaromatic amine produced a restricted ligand cavity, which triggered off the generation of a B−O−B bridge. The B−O−B bridge led to distorted conformations that satisfy the helical requirements, resulting in excellent spectroscopic and chiroptical properties. Tunable CPL with the highest luminescence dissymmetry factor (glum) of 1.3×10−3 and a CPL brightness (BCPL=11.5 M−1 cm−1) in the NIR region was achieved. This synthetic approach is expected to offer a new opportunity to chiral chemistry and increase flexibility for chiroptical tuning.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202218023-sup-0001-misc_information.pdf4.2 MB | Supporting Information |
| anie202218023-sup-0001-SI_mo220526b_sq.cif1.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. P. Riehl, F. S. Richardson, Chem. Rev. 1986, 86, 1–16;
- 1bG. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo, S. Abbate, Chirality 2016, 28, 696–707.
- 2L. Arrico, L. Di Bari, F. Zinna, Chem. Eur. J. 2021, 27, 2920–2934.
- 3
- 3aY.-P. Zhang, Y.-X. Zheng, Dalton Trans. 2022, 51, 9966–9970;
- 3bJ. Han, S. Guo, H. Lu, S. Liu, Q. Zhao, W. Huang, Adv. Opt. Mater. 2018, 6, 1800538;
- 3cF.-C. Kong, S.-Y. Yang, X.-J. Liao, Z.-Q. Feng, W.-S. Shen, Z.-Q. Jiang, D.-Y. Zhou, Y.-X. Zheng, L.-S. Liao, Adv. Funct. Mater. 2022, 32, 2201512.
- 4
- 4aC.-T. Lee, H.-Y. Lin, C.-H. Tsai, Opt. Express 2010, 18, 27079–27094;
- 4bC. D. Stanciu, F. Hansteen, A. V. Kimel, A. Kirilyuk, A. Tsukamoto, A. Itoh, T. Rasing, Phys. Rev. Lett. 2007, 99, 047601;
- 4cL. Xu, Y. Feng, D. Yu, Z. Zheng, X. Chen, W. Hong, Adv. Mater. Technol. 2020, 5, 2000373;
- 4dZ.-L. Gong, X. Zhu, Z. Zhou, S.-W. Zhang, D. Yang, B. Zhao, Y.-P. Zhang, J. Deng, Y. Cheng, Y.-X. Zheng, S.-Q. Zang, H. Kuang, P. Duan, M. Yuan, C.-F. Chen, Y. S. Zhao, Y.-W. Zhong, B. Z. Tang, M. Liu, Sci. China Chem. 2021, 64, 2060–2104.
- 5
- 5aY. Deng, M. Wang, Y. Zhuang, S. Liu, W. Huang, Q. Zhao, Light: Sci. Appl. 2021, 10, 76;
- 5bR. Farshchi, M. Ramsteiner, J. Herfort, A. Tahraoui, H. T. Grahn, Appl. Phys. Lett. 2011, 98, 162508;
- 5cA. Basiri, X. Chen, J. Bai, P. Amrollahi, J. Carpenter, Z. Holman, C. Wang, Y. Yao, Light: Sci. Appl. 2019, 8, 78;
- 5dL. E. MacKenzie, R. Pal, Nat. Chem. Rev. 2021, 5, 109–124.
- 6H. Kubo, T. Hirose, T. Nakashima, T. Kawai, J.-y. Hasegawa, K. Matsuda, J. Phys. Chem. Lett. 2021, 12, 686–695.
- 7C. Erker, T. Basché, J. Am. Chem. Soc. 2022, 144, 14053–14056.
- 8
- 8aJ. Zhang, L. Dai, A. M. Webster, W. T. K. Chan, L. E. Mackenzie, R. Pal, S. L. Cobb, G.-L. Law, Angew. Chem. Int. Ed. 2021, 60, 1004–1010; Angew. Chem. 2021, 133, 1017–1023;
- 8bR. Carr, N. H. Evans, D. Parker, Chem. Soc. Rev. 2012, 41, 7673–7686;
- 8cS. Petoud, G. Muller, E. G. Moore, J. Xu, J. Sokolnicki, J. P. Riehl, U. N. Le, S. M. Cohen, K. N. Raymond, J. Am. Chem. Soc. 2007, 129, 77–83;
- 8dJ. L. Lunkley, D. Shirotani, K. Yamanari, S. Kaizaki, G. Muller, J. Am. Chem. Soc. 2008, 130, 13814–13815;
- 8eM. Atzori, K. Dhbaibi, H. Douib, M. Grasser, V. Dorcet, I. Breslavetz, K. Paillot, O. Cador, G. L. J. A. Rikken, B. Le Guennic, J. Crassous, F. Pointillart, C. Train, J. Am. Chem. Soc. 2021, 143, 2671–2675.
- 9
- 9aY. Shen, C.-F. Chen, Chem. Rev. 2012, 112, 1463–1535;
- 9bW.-L. Zhao, M. Li, H.-Y. Lu, C.-F. Chen, Chem. Commun. 2019, 55, 13793–13803;
- 9cY. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao, Z. Tang, Matter 2022, 5, 837–875;
- 9dJ. Crassous, I. G. Stara, I. Stary, in Helicenes—Synthesis, Properties and Applications, Wiley, Hoboken, 2022.
10.1002/9783527829415 Google Scholar
- 10
- 10aY. Liu, Z. Ma, Z. Wang, W. Jiang, J. Am. Chem. Soc. 2022, 144, 11397–11404;
- 10bJ. K. Li, X. Y. Chen, W. L. Zhao, Y. L. Guo, Y. Zhang, X. C. Wang, A. C. H. Sue, X. Y. Cao, M. Li, C. F. Chen, X. Y. Wang, Angew. Chem. Int. Ed. 2023, 62, e202215367; Angew. Chem. 2023, 135, e202215367.
- 11
- 11aK. Dhbaibi, L. Abella, S. Meunier-Della-Gatta, T. Roisnel, N. Vanthuyne, B. Jamoussi, G. Pieters, B. Racine, E. Quesnel, J. Autschbach, J. Crassous, L. Favereau, Chem. Sci. 2021, 12, 5522–5533;
- 11bC. Duan, J. Zhang, J. Xiang, X. Yang, X. Gao, Angew. Chem. Int. Ed. 2022, 61, e202201494; Angew. Chem. 2022, 134, e202201494;
- 11cM. Krzeszewski, H. Ito, K. Itami, J. Am. Chem. Soc. 2022, 144, 862–871;
- 11dJ. Full, S. P. Panchal, J. Götz, A.-M. Krause, A. Nowak-Król, Angew. Chem. Int. Ed. 2021, 60, 4350–4357; Angew. Chem. 2021, 133, 4396–4403.
- 12
- 12aS. Míguez-Lago, I. F. A. Mariz, M. A. Medel, J. M. Cuerva, E. Maçôas, C. M. Cruz, A. G. Campaña, Chem. Sci. 2022, 13, 10267–10272;
- 12bN. J. Schuster, R. Hernández Sánchez, D. Bukharina, N. A. Kotov, N. Berova, F. Ng, M. L. Steigerwald, C. Nuckolls, J. Am. Chem. Soc. 2018, 140, 6235–6239;
- 12cX. Xiao, S. K. Pedersen, D. Aranda, J. Yang, R. A. Wiscons, M. Pittelkow, M. L. Steigerwald, F. Santoro, N. J. Schuster, C. Nuckolls, J. Am. Chem. Soc. 2021, 143, 983–991;
- 12dB. Mahlmeister, M. Mahl, H. Reichelt, K. Shoyama, M. Stolte, F. Würthner, J. Am. Chem. Soc. 2022, 144, 10507–10514;
- 12eC. M. Cruz, S. Castro-Fernández, E. Maçôas, J. M. Cuerva, A. G. Campaña, Angew. Chem. Int. Ed. 2018, 57, 14782–14786; Angew. Chem. 2018, 130, 14998–15002.
- 13
- 13aK. Dhbaibi, L. Favereau, J. Crassous, Chem. Rev. 2019, 119, 8846–8953;
- 13bX. Y. Wang, X. C. Wang, A. Narita, M. Wagner, X. Y. Cao, X. Feng, K. Müllen, J. Am. Chem. Soc. 2016, 138, 12783–12786;
- 13cT. Katayama, S. Nakatsuka, H. Hirai, N. Yasuda, J. Kumar, T. Kawai, T. Hatakeyama, J. Am. Chem. Soc. 2016, 138, 5210–5213.
- 14
- 14aI. H. Delgado, S. Pascal, A. Wallabregue, R. Duwald, C. Besnard, L. Guénée, C. Nançoz, E. Vauthey, R. C. Tovar, J. L. Lunkley, G. Muller, J. Lacour, Chem. Sci. 2016, 7, 4685–4693;
- 14bJ.-K. Li, X.-Y. Chen, Y.-L. Guo, X.-C. Wang, A. C. H. Sfue, X.-Y. Cao, X.-Y. Wang, J. Am. Chem. Soc. 2021, 143, 17958–17963;
- 14cK. Dhbaibi, P. Matozzo, L. Abella, M. Jean, N. Vanthuyne, J. Autschbach, L. Favereau, J. Crassous, Chem. Commun. 2021, 57, 10743–10746;
- 14dJ. Bosson, G. M. Labrador, S. Pascal, F.-A. Miannay, O. Yushchenko, H. Li, L. Bouffier, N. Sojic, R. C. Tovar, G. Muller, D. Jacquemin, A. D. Laurent, B. Le Guennic, E. Vauthey, J. Lacour, Chem. Eur. J. 2016, 22, 18394–18403.
- 15
- 15aL. Li, X. Pang, G. Liu, ACS Biomater. Sci. Eng. 2018, 4, 1928–1941;
- 15bB. Lefeuvre, C. A. Mattei, J. F. Gonzalez, F. Gendron, V. Dorcet, F. Riobé, C. Lalli, B. Le Guennic, O. Cador, O. Maury, S. Guy, A. Bensalah-Ledoux, B. Baguenard, F. Pointillart, Chem. Eur. J. 2021, 27, 7362–7366.
- 16
- 16aH. Lu, J. Mack, Y. Yang, Z. Shen, Chem. Soc. Rev. 2014, 43, 4778–4823;
- 16bA. Loudet, K. Burgess, Chem. Rev. 2007, 107, 4891–4932;
- 16cN. Boens, B. Verbelen, M. J. Ortiz, L. Jiao, W. Dehaen, Coord. Chem. Rev. 2019, 399, 213024;
- 16dN. Boens, V. Leen, W. Dehaen, Chem. Soc. Rev. 2012, 41, 1130–1172.
- 17H. Lu, J. Mack, T. Nyokong, N. Kobayashi, Z. Shen, Coord. Chem. Rev. 2016, 318, 1–15.
- 18
- 18aC. Maeda, K. Nagahata, T. Shirakawa, T. Ema, Angew. Chem. Int. Ed. 2020, 59, 7813–7817; Angew. Chem. 2020, 132, 7887–7891;
- 18bJ. Jiménez, C. Díaz-Norambuena, S. Serrano, S. C. Ma, F. Moreno, B. L. Maroto, J. Bañuelos, G. Muller, S. de la Moya, Chem. Commun. 2021, 57, 5750–5753;
- 18cL.-Y. Wang, Z.-F. Liu, K.-X. Teng, L.-Y. Niu, Q.-Z. Yang, Chem. Commun. 2022, 58, 3807–3810;
- 18dF. Zinna, T. Bruhn, C. A. Guido, J. Ahrens, M. Bröring, L. Di Bari, G. Pescitelli, Chem. Eur. J. 2016, 22, 16089–16098;
- 18eL. Cui, H. Shinjo, T. Ichiki, K. Deyama, T. Harada, K. Ishibashi, T. Ehara, K. Miyata, K. Onda, Y. Hisaeda, T. Ono, Angew. Chem. Int. Ed. 2022, 61, e202204358; Angew. Chem. 2022, 134, e202204358;
- 18fR. B. Alnoman, S. Rihn, D. C. O'Connor, F. A. Black, B. Costello, P. G. Waddell, W. Clegg, R. D. Peacock, W. Herrebout, J. G. Knight, M. J. Hall, Chem. Eur. J. 2016, 22, 93–96.
- 19
- 19aS. Wang, Z. Wang, W. Song, H. Gao, F. Wu, Y. Zhao, K. S. Chan, Z. Shen, Dalton Trans. 2022, 51, 2708–2714;
- 19bA. C. Y. Tay, B. J. Frogley, D. C. Ware, J. Conradie, A. Ghosh, P. J. Brothers, Angew. Chem. Int. Ed. 2019, 58, 3057–3061; Angew. Chem. 2019, 131, 3089–3093.
- 20
- 20aH. Lu, N. Kobayashi, Chem. Rev. 2016, 116, 6184–6261;
- 20bJ. Mack, N. Kobayashi, Chem. Rev. 2011, 111, 281–321;
- 20cG. T. Byrne, R. P. Linstead, A. R. Lowe, J. Am. Chem. Soc. (Resumed) 1934, 1017–1022.
10.1039/jr9340001017 Google Scholar
- 21a)T. Furuyama, T. Sato, N. Kobayashi, J. Am. Chem. Soc. 2015, 137, 13788–13791; b) Y.V. Zatsikha, L.I. Shamova, D.E. Herbert, V.N. Nemykin, Angew. Chem. Int. Ed. 2021, 60, 12304-12307. Angew. Chem. 2021, 133, 12412-12415.
- 22Deposition number 2221268 (for 4 a) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 23P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. van Eikema Hommes, J. Am. Chem. Soc. 1996, 118, 6317–6318.
- 24D. Geuenich, K. Hess, F. Köhler, R. Herges, Chem. Rev. 2005, 105, 3758–3772.
- 25H. Tanaka, Y. Inoue, T. Mori, ChemPhotoChem 2018, 2, 386–402.