Altering Oxygen Binding by Redox-Inactive Metal Substitution to Control Catalytic Activity: Oxygen Reduction on Manganese Oxide Nanoparticles as a Model System**
A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2022-wzr8v).
Graphical Abstract
Substituted manganese oxide nanoparticles, Mn3O4:M, were studied as a model system to generalize the electronic effect of redox-inactive substituents on the electrocatalytic activity of the oxides. The oxygen reduction activity of Mn3O4:M correlates with the enthalpy of formation of the binary MO oxide and the Lewis acidity of the M2+ site. Our work provides a perspective on the design of new compositions for oxygen electrocatalysis.
Abstract
Establishing generic catalyst design principles by identifying structural features of materials that influence their performance will advance the rational engineering of new catalytic materials. In this study, by investigating metal-substituted manganese oxide (spinel) nanoparticles, Mn3O4:M (M=Sr, Ca, Mg, Zn, Cu), we rationalize the dependence of the activity of Mn3O4:M for the electrocatalytic oxygen reduction reaction (ORR) on the enthalpy of formation of the binary MO oxide, ΔfH°(MO), and the Lewis acidity of the M2+ substituent. Incorporation of elements M with low ΔfH°(MO) enhances the oxygen binding strength in Mn3O4:M, which affects its activity in ORR due to the established correlation between ORR activity and the binding energy of *O/*OH/*OOH species. Our work provides a perspective on the design of new compositions for oxygen electrocatalysis relying on the rational substitution/doping by redox-inactive elements.
Introduction
Increasing the penetration of renewable energy production and storage technologies in energy systems is one of the major global challenges.1-3 One of the most crucial elements towards this goal is the development of efficient catalysts for electrochemical energy conversion processes such as the oxygen reduction reaction (ORR) and the reverse oxygen evolution reaction (OER), which are defining the efficiencies of water-splitting electrolyzers, fuel cells and metal-air batteries.4-12 Yet, despite decades of research in this area, even the best known catalysts operate at relatively high overpotentials. The origin of this limited efficiency stems from scaling relations between the free energies of key ORR/OER intermediates and transition states that cause them to change in a concerted manner in response to variations in the catalyst structure. Hence, even an optimum balance between adsorption energies inevitably yields a reaction overpotential.13-17 Importantly, these scaling relations (i.e., strong linear correlations between the adsorption energies of the intermediates of the catalytic cycle) are fundamental properties of the reaction intermediates sharing the same binding site and are independent of the nature of the catalyst surface.18 Extending the descriptor approach (i.e., defining a singular physical property of the material correlating with its catalytic activity) beyond correlations based on the adsorption energies of the reaction intermediates (which are derived from theoretical calculations and are difficult to assess experimentally), relationships between the bulk properties of the material and its surface reactivity have also been proposed. This has led to the introduction of a series of descriptors correlating electronic band structure features,19-21 electron count,22 molecular orbital occupancy,23, 24 redox and thermochemical properties25-29 to the surface reactivity of the electrocatalyst.
Despite these advances in the fundamental understanding of structure–activity relationships in oxygen electrocatalysis, the above properties defining the catalytic activity (activity descriptors) are typically applicable to only a restricted set of material families, and/or correlate the catalytic activity with properties (e.g., O 2p band center, charge transfer energy, adsorption energy of O2, etc.), which are complex, convoluted functions of the bulk structure of a catalyst. Therefore, establishing a structural feature of a material, which can be tailored in a straightforward manner to directly impact the catalyst performance, would constitute a major step towards the rational engineering of advanced catalysts for oxygen electrocatalysis.
In this study, we set out to rationalize the effect of the incorporation of redox-inactive elements in the structure of metal oxides on their activity in oxygen electrocatalysis. Mn3O4 was selected as a model catalyst system due to its high activity in oxygen reduction and the reported stability of the bulk Mn3O4 stoichiometry under ORR potentials, at least over relatively short times.30 We established that the activity of M-substituted Mn3O4:M (M=Sr, Ca, Mg, Zn, Cu) nanoparticles in ORR can be varied by nearly one order of magnitude by incorporation of as little as ca. 2.5 at.% of M into the structure of Mn3O4; the substitution of Mn by Sr, Ca or Mg increases the ORR activity, while the incorporation of Zn or Cu results in a decrease of the ORR activity with respect to unsubstituted Mn3O4. The observed trends with regard to Mn substitution by M are due to the correlation between the enthalpy of formation of the binary oxide MO (ΔfH°(MO)) and the binding energies of ORR intermediates (*O/*OH/*OOH species) on the catalyst surface, which have been established in previous studies as a descriptor for the catalytic activity of metal oxides in oxygen redox reactions.13, 14, 17 Our finding provides an effective tool for tailoring the catalytic properties of catalysts for ORR (and presumably for OER): the introduction of redox-inactive metal substituents/dopants into the host metal oxide catalyst enables a fine tuning of the binding energies of reaction intermediates, which define the position of the catalyst in the ORR/OER activity volcano diagram.31 We speculate this principle is general and applicable to various oxide/hydroxide families and processes concerning oxygen adsorption and/or redox reactions involving oxygen.
Results and Discussion
Synthesis and Characterization of Mn3O4:M Nanoparticles
To study the effect of the incorporation of redox-inactive metal substituents into oxides on their activity for ORR, we have selected the Mn3O4 oxide family as a model system owing to the ability of the spinel structure to readily accommodate different elemental substitutions,32 the high activity of Mn3O4 for ORR,30, 33 and the fact that the bulk Mn3O4 stoichiometry does not undergo noticeable changes at ORR potentials, at least over relatively short times.30 The metal substituents for the Mn3O4:M (Figure 1a) series (M2+=Sr2+, Ca2+, Mg2+, Zn2+, Cu2+) were selected to cover elements with pKa([M(H2O)n]2+) and enthalpies of MO oxide formation (ΔfH°) (see Table S1 in the Electronic Supporting Information, ESI) that exceed and fall below the respective values of Mn2+ (i.e., pKa([Mn(H2O)n]2+) and ΔfH°(MnO)). The Mn3O4:M nanoparticles were prepared by colloidal synthesis following a literature procedure34 using a nominal mass loading of 2.5 at.% of the M substituent with respect to the manganese content. For comparison, Mn3O4:Sr nanoparticles with a higher loading (i.e., 5 at.% of Sr) were also synthesized. Note that although increasing concentrations of substituent will likely have even stronger impact on the activity, higher substituent concentrations were not explored in Mn3O4:M series, as in this case the synthesis yielded particles of irregular size and shape distribution that would not allow for the reliable comparison of intrinsic activities and tracking of the reactivity trends, which was the main goal of this study (rather than finding the optimum composition). Barium substitution (Ba2+ is the only M2+ ion with basicity exceeding that of Sr2+[35]) was not investigated due to the large mismatch of the ionic sizes of Ba2+ and Mn2+ making incorporation of Ba2+ into Mn3O4 lattice challenging. Below, we focus on a detailed structural analysis of Mn3O4:Sr and Mn3O4:Cu, as these compositions demonstrate the highest and lowest ORR activity, respectively, within the Mn3O4:M series.
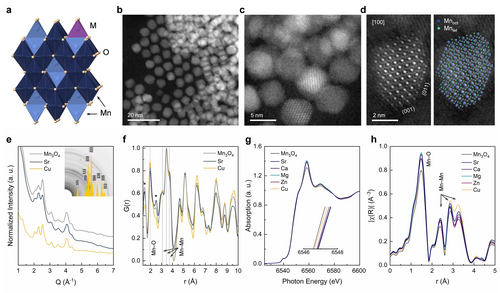
Characterization of the Mn3O4:M series (M=Sr, Ca, Mg, Zn, Cu). (a) Crystal structure of Mn3O4:M. (b–d) Representative ADF-STEM images of Mn3O4:M nanoparticles (M=Cu [b, c], Sr [d]) demonstrating a uniform size distribution and well-defined crystallinity. (d) Left panel: atomic-resolution ADF-STEM image along the <100>-zone axis; the brightest atomic columns are due to Mn atoms in octahedral positions (Mnoct) with 100 % occupancy. Right panel: atomistic crystallographic model of the spinel, superimposed onto the ADF-STEM image shown on the left panel; large blue circles correspond to Mnoct with 100 % occupancy, small blue circles are due to Mnoct with 50 % occupancy, green circles represent Mn in tetrahedral positions (Mntet). (e) one dimensional selected area electron diffraction pattern (SAED) obtained by rotational averaging of the two-dimensional diffraction pattern of Mn3O4:M nanoparticles deposited onto a grid from a hexane suspension. The inset confirms that the calculated electron diffraction pattern (Mn3O4, COD 1514236) matches the experimental pattern evidencing phase purity and the absence of noticeable effects of the substituent M on the crystallographic structure. (f) Electron PDF analysis of Mn3O4:M showing the same short order structure in the Mn3O4:M series (M=Sr, Cu) irrespective of M. Peaks marked with asterisks are due to amorphous carbon. (g) Normalized Mn K-edge XANES and (h) phase-uncorrected Fourier transform of the Mn K-edge EXAFS function for the Mn3O4:M materials. Labels on the plots refer to the specific M in the Mn3O4:M series.
Annular dark-field scanning transmission electron microscopy (ADF-STEM) and high-resolution transmission electron microscopy (HR-TEM) imaging of the as-synthesized nanoparticles (Figure 1b–d) revealed their high monodispersity with an average particle size of the different M substituted nanoparticles in the range 3–5 nm (Figure S1, Table S2). Figure 1d (left panel) shows an atomic resolution ADF-STEM image of a Mn3O4:Sr nanocrystal oriented along the <100> zone axis. The bright spots correspond to atomic columns of Mn and Sr, whereas the oxygen sub-lattice was not registered by the ADF detector due to its low scattering power and the different angular distribution of the scattered electrons. Direct identification of the Sr sites proved to be challenging, as we could not observe any noticeable contrast change in the local atomic rows corresponding to the M2+ sites due to the low amount of Sr incorporated and its high dispersion. The atomic arrangement is consistent with a spinel model for the cation sub-lattice as visualized by the overlay of the ADF-STEM image with the respective structural model (Figure 1d, right panel). Along the <100> zone axis, the spinel structure contains close-packed planes with [MnO6] octahedral (Mnoct) layers alternating with mixed [MnO6] and [MnO4] tetrahedral (Mntet) layers. The detailed ADF-STEM analysis of the particle surface (Figure 1d, S2) revealed that the majority of the terminating layers were composed exclusively of Mnoct. Similar terminations of closed packed layers with cations in octahedral positions have been observed previously for several isostructural systems, such as γ-Ga2O3, γ-Fe3O4 and γ-Al2O3.36-38 In line with experimental observations, DFT calculations (see discussion below) indicate that the surface terminated by Mnoct is thermodynamically the most stable surface.
X-ray diffraction (XRD, Figure S3) and electron diffraction (Figure 1e) patterns also confirm the presence of a nanocrystalline spinel structure and the absence of any detectable amounts of secondary phases. The low content of M incorporated into the spinel structure (ca. 2.5 at.% from inductively coupled plasma (ICP) spectroscopy analysis, see Table S2) precludes a noticeable shift of the diffraction peaks. In addition, energy dispersive X-ray spectroscopy (EDX) of the sample Mn3O4:Sr evidenced a homogeneous distribution of the elements in the synthesized material (Figure S4), providing further evidence for the successful incorporation of M2+ ions into the Mn3O4 spinel structure. Additionally, Raman spectroscopy that is sensitive to variations of the manganese oxides composition39 confirmed the formation of the Mn3O4 spinel structure (Figure S5).
A comparison of the pair distribution functions (PDF) obtained from electron diffraction data is shown in Figure 1f. The PDF peaks located at 1.44 Å and 2.48 Å are due to the presence of an amorphous carbon film in the TEM sample grid. The first pair correlation peak at ca. 2 Å is due to Mn−O distances, the second PDF peak at ca. 3 Å is due to edge-sharing Mn3+−Mn3+octahedra, while the third and the fourth PDF peaks (3.45 Å and 3.75 Å) are mainly due to the contribution from Mn−Mn corner-sharing polyhedra. Notably, the PDF peak positions and their intensities ratio are the same for Mn3O4, Mn3O4:Sr, and Mn3O4:Cu indicating that the local atomic arrangement remained unperturbed by the substitution of Mn by M.
X-ray absorption spectroscopy (XAS) on the Mn K-edge was applied to probe the average oxidation state and local environment of the manganese sites in Mn3O4:M. The shape of the white line in the X-ray absorption near edge structure (XANES) spectrum of Mn3O4:M nanoparticles showed only slight differences when compared to that of the commercial bulk Mn3O4 reference (Figure S6). We observed a small yet distinguishable (ca. 0.2 eV) shift of the position of the absorption edge towards high energies for all M-substituted Mn3O4:M materials with respect to the unsubstituted Mn3O4 nanoparticles, as well as the rise of the white line upon substitution (Figure 1g), consistent with the replacement of Mn2+ sites in the spinel structure with M2+ ions and an associated increase of the average oxidation state of manganese (owing to an increased ratio of Mn3+:Mn2+ sites). In line with this argument, the smallest change in the edge position and increase in the intensity of the white line was observed for the Mn3O4:Cu sample that contained the smallest quantity of the substituent in Mn3O4:M series (1.0 at.%, Table S2). A slight decrease in the pre-edge peak for the M-substituted materials suggests an increase in the average symmetry of the sites, possibly due to the increased fraction of Mntet sites (that are less distorted than the Mnoct sites).
Extended X-ray absorption fine structure (EXAFS) analysis was used to probe the local environment of the manganese sites in the Mn3O4:M materials (Figure 1h, S7). The fitting of the experimental EXAFS data (Figure 1h) for the Mn3O4:M series is in good agreement with the crystallographic model of the Mn3O4 spinel40 (Figure 1a) and our PDF analysis (Figure 1e). The structural model consists of two Mn sites, in tetrahedral (Mntet) and octahedral (Mnoct) coordination with O, which were used to generate two series of scattering paths. Fixing the coordination numbers, allowed us to determine the Mn−O (within the polyhedra) and Mn−Mn (between the polyhedra) distances. The first coordination shell is composed of three Mn−O scattering paths corresponding to Mntet−O and two non-equivalent Mnoct−O subshells that are due to the highly distorted octahedra, while the second sphere is dominated by Mnoct−Mnoct and Mnoct−Mntet paths (Table S3, Figure S7). Although, the element substitution is expected to affect the covalency of the Mn−O bond (hence also the bond length) due to the inductive effect of M,41 structural changes associated with the substitution of Mn by M are likely too subtle to be traced by EXAFS analysis: indeed, for the entire Mn3O4:M series, all Mn−O bond lengths are virtually undistinguishable within experimental uncertainty (0.01–0.03 Å). Similarly, small changes in the XANES caused by the substitution of Mn2+by M2+ (i.e., shift of the edge position) make it challenging to quantify the effect of differences in the electron withdrawing strength of M on the electronic states of manganese. In line with the Mn K-edge XAS analysis, Mn 2p and Mn 3p core levels X-ray photoelectron spectroscopy (XPS) (Figure S8, S9) and O K-edge XAS spectroscopy (Figure S10) do not reveal any pronounced effect of substituent M in Mn3O4:M on the Mn states.
Despite the pronounced stability of the bulk Mn3O4 structure, evidenced by Mn K-edge XAS measurements,30 some studies report on the instability of Mn3O4 surface during electrochemical cycling at ORR potentials in harsh alkaline environment,43 as well as effect of Nafion binder used for the preparation of the catalyst inks on the Mn electronic states.44 However, for Mn3O4:M materials studied here, XPS analysis of the samples deposited from the Nafion-containing inks did not evidence any noticeable differences in the envelope of the Mn 3p core level region compared to the pristine samples (Figure S9) indicating low reactivity of Mn3O4:M towards Nafion. Interestingly, Mn2+/Mn3+/Mn4+ ratio (oxidized Mn4+ species are present on the surface of all as-synthesized Mn3O4:M materials, in line with other reports on XPS analysis of Mn3O4 oxides43, 45) did not vary over 20 CV cycles (0.4 VRHE–1.0 VRHE, 100 mV s−1, 0.1 M KOH) (Figure S9, Table S4), providing evidence for the high stability of the spinel structure over the period of the catalytic measurements. Selected area electron diffraction (SAED) patterns of the Mn3O4:M materials after potential cycling (Figure S11) confirm the presence of a nanocrystalline spinel structure and the absence of any detectable amounts of secondary crystalline phases, whereas TEM analysis (Figure S12) does not reveal any noticeable changes in particles morphology, size, or crystallinity indicating that surface changes (if any) can only be confined to surface atomic layer(s). (The long-term stability of Mn3O4:M is however an issue as the ORR activity reduces significantly after 1000 CV cycles, Figure S13).
XPS measurements of the O 1s core levels allowed us to confirm the inductive effect of M as evidenced by a shift of the binding energy of the lattice oxygen component (by ca. 0.2 eV) to lower binding energies when comparing Mn3O4:Sr to Mn3O4:Cu (Figure S14). This shift is indicative of a larger negative charge on the oxygen sites in Mn3O4:Sr compared to Mn3O4:Cu, which is in line with an increased electron donating strength (i.e., a reduced Lewis acidity) of strontium with respect to copper (the trend holds well for the entire substitution series, Figure S15).
Overall, our detailed structural characterizations of the series of Mn3O4:M nanoparticles demonstrate the formation of phase-pure, M-substituted Mn3O4 oxides. The incorporation of M had a negligible effect on the crystal structure, the nanoparticle morphology and the manganese valence states, making this series of materials a reliable model system to decouple and investigate the individual effect of the different M substituents on the electrocatalytic activity of the material.
Oxygen Reduction Activity of the Mn3O4:M Series
The ORR activity of the series of Mn3O4:M nanoparticles was assessed in a conventional three-electrode electrochemical setup using 0.1 M KOH as the electrolyte, whereas the H2O2/H2O selectivity was estimated using rotating ring-disk electrode measurements (RRDE, see Supporting Information for the experimental details). Intrinsic ORR activities were assessed using steady-state galvanostatic measurements (to eliminate the contribution of redox processes within the bulk of the electrode material, which can interfere with the measured catalytic currents in conventional CV studies,46 Figure S16). The currents were normalized to the geometric surface area of the nanoparticles estimated by TEM (see Figure S1, Table S2). As the CV curves for Mn3O4:M samples do not feature a well-defined limiting current plateau, our analysis focused on the current range that was much smaller than the mass transport limit, where the mass transport correction can be neglected (for Tafel plots for extended current range, see Figure S17).
The Mn3O4:M nanoparticle series studied here feature mass and electrode surface area normalized activities (Figure S18) that are comparable to previously reported activities of MnOx catalysts.47 The specific activities (per oxide surface area) are similar to other high surface area MnOx oxides (ca. 1 μA cmoxide−2 at 0.8 VRHE),48, 49 but below the activities typically reported for non-porous highly crystalline materials (>10 μA cmoxide−2 at 0.8 VRHE).47 Remarkably, we found that the measured ORR activity depends strongly on the type of the M substituent. Indeed, incorporation of 2 at.% of Sr increased the ORR activity by almost an order of magnitude compared to Mn3O4:Cu (Figure 2a).
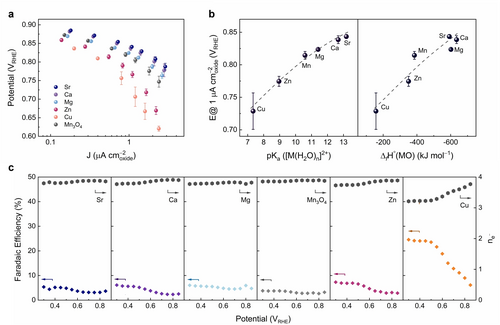
Electrocatalytic properties of Mn3O4:M. (a) Tafel plots for the ORR on Mn3O4:M nanoparticles (M=Sr, Ca, Mg, Zn, Cu) loaded onto a glassy carbon support, as obtained from galvanostatic measurements. Error bars are standard deviations from at least three independent measurements. Conditions: O2-saturated 0.1 M KOH, 1600 rpm, oxide loading 0.1 mg cm−2; no mass transport correction was applied. (b) Scaling between the ORR activity given as the potential to achieve a current density of 1 μA cm−2 (normalized to the oxide surface area estimated by TEM measurements, see Table S2) and pKa ([M(H2O)n]2+)35 (measure of Lewis acidity) and the experimental enthalpy of formation of the binary oxide, MO, ΔfH°(MO).42 The dashed lines are to guide the eye. (c) ORR selectivity towards H2O2 production in terms of Faradaic efficiency and ORR electron transfer number (ne-) obtained from rotating ring-disk electrode (RRDE) measurements as a function of the electrode potential. Data are obtained from galvanostatic measurements at 900 rpm. Labels on the plots refer to the specific M in the Mn3O4:M series.
Importantly, as described above, this was achieved without changing the crystal structure, particle morphology, nor the valence state of manganese. This suggests that there exist direct correlations between certain intrinsic properties of the substituting elements and the catalytic performance of Mn3O4:M. In fact, the influence of the incorporation of redox-inactive substituents on the redox properties of biological systems50 and molecular complexes has been reported51-55 and the observed trends have been rationalized typically by an inductive effect triggered by the substituent/dopant altering the covalency of the bonds between the parent metal centers and adjacent oxygen.41 Although many studies have explored the effect of metal substitution in oxides (e.g., perovskites56) on their catalytic performance, changes in the catalytic activity have been attributed typically to changes in the valence state of the active site (for aliovalent substitution),57-59 changes in the crystal structure of the oxide,60 or the introduction of crystallographic strain,61 i.e., effects originating from the mismatch of the size or the formal charge of the substituent and the substituted site (note that in this context, we do not consider systems for which the substitution results in a change of the reaction site, e.g., from Fe to Mn in MnxFe3−xO462). (Specifically for Mn3O4, metal substitution was used as a tool to tailor electrocatalytic activity.43, 63, 64) On the other hand, there are very few experimental works that aim to leverage electronic, or inductive, effects arising from the incorporation of substituents to rationally tailor the activity of electrocatalysts (examples can be found in references65-68). Hence, establishing a unified description of the effect conferred by the introduction of redox-inactive metals into a host oxide on their catalytic ORR/OER activity (irrespective of their structure) would provide a new perspective (and experimental tool) for the design of materials for targeted electrochemical transformations.
Reactivity Trends: Theoretical Considerations
Next, we aim to assess the correlation(s) between the ORR activity of the Mn3O4:M nanoparticles (Figure 2a) and the properties of the substituting element M. First, we observed a strong correlation between the ORR activity of Mn3O4:M and the Lewis acidity of the substituting M2+ ions (expressed as pKa of the corresponding hydrated ion, [M(H2O)n]2+, Figure 2b), implying that the Mn−O bond covalency (tuned by the inductive effect of M) controls the catalytic activity of the material. As discussed above, XPS O 1s core level measurements confirmed the inductive effect of M reflected in a larger negative charge on the oxygen sites in Mn3O4:Sr compared to Mn3O4:Cu owing to the greater electron donating strength (i.e., a reduced Lewis acidity) of strontium with respect to copper. This robust correlation between the ORR activity and the pKa([M(H2O)n]2+) provides clear evidence for the relevance of the Lewis acidity of the redox-inactive substituent on the electrocatalytic activity of Mn3O4 (note that in a related case, the oxygen exchange kinetics of Pr0.1Ce0.9O2-δ was also shown to depend on the acidity [on the Smith scale] of the surface-infiltrated oxides species,69 however the catalytic activities in ORR/OER reported for a wide row of oxides did not demonstrate a correlation to the Smith acidities of the respective oxides70). Thus, to construct a clear and direct link between the properties of M and the catalytic activity of an oxide in terms related to the mechanism of oxygen electrocatalysis, we further analyzed the activity and selectivity (Figure 2c) trends.
As the ORR mechanism includes the formation/cleavage of metal-oxygen bonds (whereby their binding strength controls the catalytic activity),13 it is expected that the energy of oxygen vacancy formation [E(Ovac)] also scales with catalytic activity.29 Indeed, the calculated values for E(Ovac) (for O atoms bound to octahedrally coordinated Mn atoms serving as the active sites in spinel oxides71) in the vicinity of M in a Mn3O4:M model slab (Figure 3a) demonstrate a good correlation with the experimentally measured activities (Figure 3b), hence confirming a relationship between the catalytic activity and the oxygen binding strength on the catalyst surface. As a consequence, we observe an excellent correlation between the ORR activity of Mn3O4:M nanoparticles and the experimental enthalpy of formation of the binary oxide, MO (ΔfH°(MO), Figure 2b), which is rooted in the dependence of the binding strength of the oxygen species on the oxide surface on ΔfH°(MO) (Figure 3c): incorporation of an element with a more negative ΔfH°(MO) (i.e., a thermodynamically more stable oxide) increases the binding strength of oxygen species (*O/*OH/*OOH) on the surface of the multimetallic compound (Mn3O4:M). These findings are fundamentally distinct from the correlations involving oxide formation energies of metals representing the actual redox-active site discussed for binary oxides25 or single-atom catalysts.72, 73
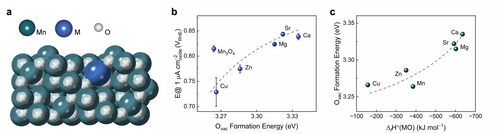
DFT studies of the Mn3O4:M series. (a) Structural model featuring a Mnoct terminated surface (in agreement with experimental observations, Figure 1d) employed to calculate properties of Mn3O4:M using DFT. (b) Relationship between experimentally determined specific ORR activity and the DFT-calculated formation energy of oxygen vacancies (Ovac). Error bars are standard deviations from at least three independent measurements (see Experimental section in ESI for details). (c) Relationship between the energy of Ovac formation and the experimental enthalpy of formation of the MO, ΔfH°(MO).42 Labels on the plots refer to the specific M in the Mn3O4:M series.
The correlations established here demonstrate that the substitution/doping by redox-inactive elements can be used as a highly versatile tool to rationally tune the activity of complex oxide/hydroxide catalysts for ORR (and likely for the reverse OER), whereby the most appropriate choice of the substituent/dopant depends on the rate-limiting step of the catalytic reaction. Specifically for the commonly quoted associative mechanism of the oxygen reduction reaction,13 the reaction rate is limited by the adsorption of either *OOH (in case of weak oxygen binding) or *OH (strong oxygen binding).74 Therefore, in the former scenario, the incorporation of elements M with a highly negative value of ΔfH°(MOx) would result in an increase of the oxygen binding strength (Mn−O), hence an activity enhancement would be expected. As shown previously, ORR on a Mn3O4 surface is indeed limited by *OOH adsorption (the least exothermic step at U=0),75 implying that an increase of the oxygen adsorption strength would improve its activity. Our experimental results for the Mn3O4:M system (with M=Sr, Ca, Mg) confirm this rationale. Note that as little as 2.5 at.% of the substituent was sufficient to yield an increase in ORR activity by an order of magnitude (Mn3O4:Sr compared to Mn3O4:Cu); increasing the concentration of Sr substitution from 2.5 at.% to 5 at.% improved the activity of Mn3O4:Sr further (Figure S19). (As mentioned above, materials with higher concentrations of M were not studied, as in this case the synthesis yielded particles of irregular shape and size distribution.) As a likely indication of the general nature of trends observed for the Mn3O4:M family, analysis41 of the literature data on the ORR activity of MxFe3−xO4,62 MCo2O4,71 and LnMnO376 materials revealed similar linear correlations between ORR activity of the complex oxides and properties of M substituent (specifically pKa([M(H2O)z]n+), as shown on Figure S20 (note those trends are not discussed in the original62, 71, 76 experimental reports). Furthermore, our calculations on a simple MnO2:M model structure (two-dimensional birnessite layer) provide a further indication that oxygen binding energy in complex oxides correlates with thermodynamic characteristics (e.g., (ΔfH°(MOx)) of the substituent/dopant, independent of the particular structure of the parent oxide (Figure S21). Yet, further studies on model systems would be desirable to convincingly demonstrate the universality of the effect of the redox-inactive substituent on the electrocatalytic performance of the complex oxide materials.
Interestingly, the substitution of Mn by M does not only affect the ORR activity, but also the H2O2/H2O (2e−/4e−) selectivity. Indeed, the Faradaic efficiency towards the 2e− reduction (H2O2 synthesis) increased from ca. 5 % for M=Sr, Ca, Mg, Mn (catalytically more active oxides in the Mn3O4:M series) to ca. 8 % for M=Zn, and ca. 25 % for M=Cu at high overpotentials (Figure 2c). Such a selectivity trend was predicted for ORR catalysts characterized by a weak oxygen binding strength (i.e., the reaction is limited by O2 adsorption to form *OOH, as in the case of Mn3O475), whereas a further decrease of the adsorption energy was proposed to enhance the 2e− reduction selectivity.77 This experimental observation provides additional evidence for the interplay between the properties of the redox-inactive substituent (e.g., ΔfH°(MOx)), the adsorption strength of catalytic intermediates (*O/*OH/*OOH), and the ORR activity and selectivity.
General Implications for Catalysts Design
Despite its simplicity, the concept described here (incorporation of redox-inactive elements into the structure of oxide to leverage their inductive effect) has not been studied systematically or considered as a general strategy for the rational design of oxide electrocatalysts. While previous studies probing correlations between the thermochemical properties of bulk oxides and their activity in ORR/OER electrocatalysis25, 26, 29 attempted to explain the reactivity trends for certain oxide families, our findings can be used to instruct the design of catalyst across different material families by the manipulation of their composition through the incorporation of substituent/dopant. It should be noted here that the incorporation of substituents/dopants into the lattice of the host oxide may introduce multiple effects, such as the generation of crystal lattice strain and distortion, modification of the crystal structure, change of spin or the valence state of the active site, etc., which can have counteracting effects on the catalytic activity, and therefore have to be considered as well. Yet, for a large variety of oxide families that are capable of accommodating a broad range of cationic species without leading to noticeable structural changes, and particularly for structurally non-rigid metal (oxyhydr)oxides, the concept introduced here offers a potent tool for the design of highly active materials for oxygen electrocatalysis, and can be applied to engineer compositions with benchmark performances starting from current state-of-the-art materials (e.g., RuO2/IrO2/Ni−Fe oxyhydroxides, etc.), however, this is yet to be demonstrated. Further work will attempt to verify whether this principle is general and can be transferred to a broader selection of materials and processes concerning oxygen adsorption and redox reactions involving oxygen, reaching beyond electrocatalysis, such as metal-ion batteries,78 chemical looping,79 or heterogeneous catalysis.80
Conclusion
By studying a series of model, metal-substituted, manganese oxide spinel nanoparticles, Mn3O4:M (M=Sr, Ca, Mg, Zn, Cu), we established a direct correlation between the enthalpy of formation of the binary MO oxide (ΔfH°(MO)), the Lewis acidity of the M2+ substituent (expressed as pKa of [M(H2O)n]2+ ion) and the activity (and selectivity) of the Mn3O4:M family in the oxygen reduction reaction. Incorporation of elements with a low (highly negative) ΔfH°(MO) enhances the oxygen binding strength in Mn3O4:M, as confirmed by XPS and DFT calculations (revealing a strong correlation between the experimental ΔfH°(MO) values and the energy of oxygen vacancy formation E(Ovac) on the Mn3O4:M surfaces). This implies that there is also a correlation between ΔfH°(MO) and the binding energies of oxygen intermediates (*O/*OH/*OOH) on the catalyst surface, which serve as key descriptors for ORR activity. Our observations concerning the effect of M substitution of Mn3O4 on its ORR activity demonstrate that the introduction of redox-inactive metal substituents/dopants into the host structure of a metal oxide is a highly versatile approach to tune the binding strength of reaction intermediates (determining the position of the catalyst on the ORR/OER activity volcano), and in turn improve the ORR/OER activity and also its selectivity. Further work will target to investigate if this principle is general and can be employed to tailor the properties of different oxide materials for diverse applications, beyond electrocatalysis, associated with oxygen adsorption and/or redox reactions involving oxygen.
Acknowledgments
The works was supported by the Swiss National Science Foundation (grant N° 196943) and the European Research Council (grant N° 819573). The authors thank ScopeM (ETH Zürich) for access to the electron microscopy facilities. Dr. Maarten Nachtegaal and Dr. Camelia Borca are acknowledged for the help with XAS measurements. The authors thank the Paul Scherrer Institute for the providing the access to the synchrotron radiation facilities at the SuperXAS and PHOENIX beamlines of the SLS. J.A.Y. and P.V.K. acknowledge the computational support from National Computing Infrastructure (NCI) Australia. This publication was created as part of NCCR Catalysis (grant N° 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.