I2-Catalyzed Cycloisomerization of Ynamides: Chemoselective and Divergent Access to Indole Derivatives
Bo-Han Zhu
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
These authors contributed equally to this work.
Search for more papers by this authorSheng-Bing Ye
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
These authors contributed equally to this work.
Search for more papers by this authorMin-Ling Nie
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
Search for more papers by this authorZhong-Yang Xie
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
Search for more papers by this authorYi-Bo Wang
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Peng-Cheng Qian
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Qing Sun
Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, Nanchang Hangkong University, Nanchang, 330063 China
Search for more papers by this authorProf. Dr. Long-Wu Ye
State Key Laboratory of Physical Chemistry of Solid Surfaces and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Long Li
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
State Key Laboratory of Physical Chemistry of Solid Surfaces and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorBo-Han Zhu
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
These authors contributed equally to this work.
Search for more papers by this authorSheng-Bing Ye
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
These authors contributed equally to this work.
Search for more papers by this authorMin-Ling Nie
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
Search for more papers by this authorZhong-Yang Xie
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
Search for more papers by this authorYi-Bo Wang
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Peng-Cheng Qian
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Qing Sun
Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, Nanchang Hangkong University, Nanchang, 330063 China
Search for more papers by this authorProf. Dr. Long-Wu Ye
State Key Laboratory of Physical Chemistry of Solid Surfaces and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Long Li
College of Chemistry & Materials Engineering, Wenzhou University, Wenzhou, 325035 China
Wenzhou Key Laboratory of Technology and Application of Environmental Functional Materials, Institute of New Materials & Industry Technology, Wenzhou University, Wenzhou, 325000 China
State Key Laboratory of Physical Chemistry of Solid Surfaces and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorGraphical Abstract
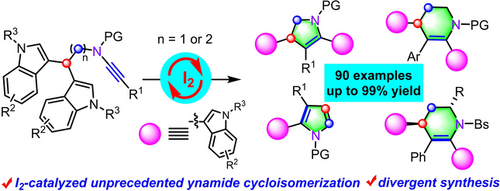
An I2-catalyzed unprecedented cycloisomerization of ynamides is developed, allowing various bis(indole) derivatives in good to excellent yields with wide substrate scope, which not only represents the first molecular iodine-catalyzed tandem complex alkyne cycloisomerizations but also constitutes the first chemoselective cycloisomerization of tryptamine-ynamides involving distinctively different C−C (alkyl) bond cleavage and rearrangement.
Abstract
Herein, an I2-catalyzed unprecedented cycloisomerization of ynamides is developed, furnishing various functionalized bis(indole) derivatives in generally good to excellent yields with wide substrate scope and excellent atom-economy. This protocol not only represents the first molecular-iodine-catalyzed tandem complex alkyne cycloisomerizations, but also constitutes the first chemoselective cycloisomerization of tryptamine-ynamides involving distinctively different C(sp3)−C(sp3) bond cleavage and rearrangement. Moreover, chiral tetrahydropyridine frameworks containing two stereocenters are obtained with moderate to excellent diastereoselectivities and excellent enantioselectivities. Meanwhile, cycloisomerization and aromatization of ynamides produce pyrrolyl indoles with high efficiency enabled by I2. Additionally, control experiments and theoretical calculations reveal that this reaction probably undergoes a tandem 5-exo-dig cyclization/rearrangement process.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202215616-sup-0001-misc_information.pdf60.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews see:
- 1aS. Kohmoto, Y. Kashman, O. J. McConnell, K. L. Rinehart, A. Wright, F. Koehn, J. Org. Chem. 1988, 53, 3116;
- 1bS. Sakemi, H. H. Sun, J. Org. Chem. 1991, 56, 4304;
- 1cS. P. Gunasekera, P. J. McCarthy, M. Kelly-Borges, J. Nat. Prod. 1994, 57, 1437;
- 1dX.-H. Gu, X.-Z. Wan, B. Jiang, Bioorg. Med. Chem. Lett. 1999, 9, 569;
- 1eA. Casapullo, G. Bifulco, I. Bruno, R. Riccio, J. Nat. Prod. 2000, 63, 447;
- 1fW.-N. Xiong, C.-G. Yang, B. Jiang, Bioorg. Med. Chem. 2001, 9, 1773;
- 1gK.-B. Oh, W. Mar, S. Kim, J.-Y. Kim, M.-N. Oh, J.-G. Kim, D. Shin, C. J. Sim, J. Shin, Bioorg. Med. Chem. Lett. 2005, 15, 4927.
- 2For selected reviews, see:
- 2aD. Campeau, R. Leon, F. David, A. Mansour, K. Muratov, F. Gagosz, Chem. Rev. 2021, 121, 8756;
- 2bS. A. Blaszczyk, D. A. Glazier, W. Tang, Acc. Chem. Res. 2020, 53, 231;
- 2cD. Qian, J. Zhang, Acc. Chem. Res. 2020, 53, 2358;
- 2dW. Zhao, J. Sun, Chem. Rev. 2018, 118, 10349;
- 2eZ. Zheng, Z. Wang, Y. Wang, L. Zhang, Chem. Soc. Rev. 2016, 45, 4448;
- 2fW. Zi, F. D. Toste, Chem. Soc. Rev. 2016, 45, 4567;
- 2gA. M. Asiri, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 4471;
- 2hV. K. Tiwari, E. Aguilar, R. Sanz, M. A. Fernández-Rodríguez, P. García-García, Chem. Rev. 2016, 116, 8256;
- 2iD. Qian, J. Zhang, Chem. Soc. Rev. 2015, 44, 677;
- 2jR. Dorel, A. M. Echavarren, Chem. Rev. 2015, 115, 9028;
- 2kC. Obradors, A. M. Echavarren, Acc. Chem. Res. 2014, 47, 902;
- 2lA. S. K. Hashmi, Acc. Chem. Res. 2014, 47, 864;
- 2mR. Chinchilla, C. Najera, Chem. Rev. 2014, 114, 1783;
- 2nX.-Z. Shu, D. Shu, C. M. Schienebeck, W. Tang, Chem. Soc. Rev. 2012, 41, 7698;
- 2oK. Gilmore, I. V. Alabugin, Chem. Rev. 2011, 111, 6513;
- 2pC. Aubert, L. Fensterbank, P. Garcia, M. Malacria, A. Simonneau, Chem. Rev. 2011, 111, 1954.
- 3For selected examples, see:
- 3aZ. L. Niemeyer, S. Pindi, D. A. Khrakovsky, C. N. Kuzniewski, C. M. Hong, L. A. Joyce, M. S. Sigman, F. D. Toste, J. Am. Chem. Soc. 2017, 139, 12943;
- 3bP. T. Bohan, F. D. Toste, J. Am. Chem. Soc. 2017, 139, 11016;
- 3cW. Zi, H. Wu, F. D. Toste, J. Am. Chem. Soc. 2015, 137, 3225;
- 3dH. Wu, W. Zi, G. Li, H. Lu, F. D. Toste, Angew. Chem. Int. Ed. 2015, 54, 8529; Angew. Chem. 2015, 127, 8649.
- 4For selected examples, see:
- 4aA. Franchino, À. Martí, A. M. Echavarren, J. Am. Chem. Soc. 2022, 144, 3497;
- 4bI. Martín-Torres, G. Ogalla, J. M. Yang, A. Rinaldi, A. M. Echavarren, Angew. Chem. Int. Ed. 2021, 60, 9339; Angew. Chem. 2021, 133, 9425;
- 4cG. Zuccarello, J. G. Mayans, I. Escofet, D. Scharnagel, M. S. Kirillova, A. H. Pérez-Jimeno, P. Calleja, J. R. Boothe, A. M. Echavarren, J. Am. Chem. Soc. 2019, 141, 11858.
- 5For selected examples, see:
- 5aR. Heckershoff, T. Schnitzer, T. Diederich, L. Eberle, P. Krämer, F. Rominger, M. Rudolph, A. S. K. Hashmi, J. Am. Chem. Soc. 2022, 144, 8306;
- 5bC. Hu, T. Wang, M. Rudolph, T. Oeser, A. M. Asiri, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2020, 59, 8522; Angew. Chem. 2020, 132, 8600;
- 5cT. Wurm, J. Bucher, S. B. Duckworth, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2017, 56, 3364; Angew. Chem. 2017, 129, 3413.
- 6For selected examples, see:
- 6aK. Zhao, P. Kohnke, Z. Yang, X. Cheng, S.-L. You, L. Zhang, Angew. Chem. Int. Ed. 2022, 61, e2022075188; Angew. Chem. 2022, 134, e2022075188;
- 6bX. Cheng, Z. Wang, C. D. Quintanilla, L. Zhang, J. Am. Chem. Soc. 2019, 141, 3787;
- 6cB. Lu, Y. Li, Y. Wang, D. H. Aue, Y. Luo, L. Zhang, J. Am. Chem. Soc. 2013, 135, 8512.
- 7For selected examples, see:
- 7aA. Pommainville, D. Campeau, F. Gagosz, Angew. Chem. Int. Ed. 2022, 61, e202205963; Angew. Chem. 2022, 134, e202205963;
- 7bD. Campeau, A. Pommainville, F. Gagosz, J. Am. Chem. Soc. 2021, 143, 9601;
- 7cZ. Cao, F. Gagosz, Angew. Chem. Int. Ed. 2013, 52, 9014; Angew. Chem. 2013, 125, 9184.
- 8For selected examples, see:
- 8aR. Wu, J. Lu, T. Cao, J. Ma, K. Chen, S. Zhu, J. Am. Chem. Soc. 2021, 143, 14916;
- 8bL. Zhang, T. Cao, H. Jiang, S. Zhu, Angew. Chem. Int. Ed. 2020, 59, 4670; Angew. Chem. 2020, 132, 4700;
- 8cD. Zhu, L. Chen, H. Zhang, Z. Ma, H. Jiang, S. Zhu, Angew. Chem. Int. Ed. 2018, 57, 12405; Angew. Chem. 2018, 130, 12585;
- 8dD. Zhu, J. Ma, K. Luo, H. Fu, L. Zhang, S. Zhu, Angew. Chem. Int. Ed. 2016, 55, 8452; Angew. Chem. 2016, 128, 8592.
- 9For selected examples, see:
- 9aP.-C. Zhang, Y.-L. Li, J. He, H.-H. Wu, Z. Li, J. Zhang, Nat. Commun. 2021, 12, 4609;
- 9bT.-D. Tan, X.-Q. Zhu, H.-Z. Bu, G.-C. Deng, Y.-B. Chen, R.-S. Liu, L.-W. Ye, Angew. Chem. Int. Ed. 2019, 58, 9632; Angew. Chem. 2019, 131, 9734;
- 9cT. Namba, S. Kawauchi, Y. Shibata, H. Kanno, K. Tanaka, Angew. Chem. Int. Ed. 2017, 56, 3004; Angew. Chem. 2017, 129, 3050;
- 9dL. Huang, H.-B. Yang, D.-H. Zhang, Z. Zhang, X.-Y. Tang, Q. Xu, M. Shi, Angew. Chem. Int. Ed. 2013, 52, 6767; Angew. Chem. 2013, 125, 6899.
- 10For selected reviews, see:
- 10aT. Aggarwal, S. Kumar, A. K. Verma, Org. Biomol. Chem. 2016, 14, 7639;
- 10bY.-M. Ren, C. Cai, R.-C. Yang, RSC Adv. 2013, 3, 7182;
- 10cB. Godoi, R. F. Schumacher, G. Zeni, Chem. Rev. 2011, 111, 2937.
- 11For selected examples, see:
- 11aZ. Tang, F. Zhang, T. Yao, X.-S. Liu, Y. Liu, L. Liu, J. Org. Chem. 2022, 87, 7531;
- 11bY.-F. Qiu, Y.-J. Niu, X.-R. Song, X. Wei, H. Chen, S.-X. Li, X.-C. Wang, C. Huo, Z.-J. Quan, Y.-M. Liang, Chem. Commun. 2020, 56, 1421;
- 11cQ. Li, L. Yu, Y. Wei, M. Shi, J. Org. Chem. 2020, 85, 2438;
- 11dQ. Li, L. Yu, Y. Wei, M. Shi, J. Org. Chem. 2019, 84, 9282;
- 11eH.-T. Zhu, X. Dong, L.-J. Wang, M.-J. Zhong, X.-Y. Liu, Y.-M. Liang, Chem. Commun. 2012, 48, 10748;
- 11fD. Fischer, H. Tomeba, N. K. Pahadi, N. T. Patil, Y. Yamamoto, Angew. Chem. Int. Ed. 2007, 46, 4764; Angew. Chem. 2007, 119, 4848.
- 12For selected examples, see:
- 12aY. Wang, S. Li, X. Wang, Y. Yao, L. Feng, C. Ma, RSC Adv. 2022, 12, 5919;
- 12bN. Mukherjee, T. Chatterjee, J. Org. Chem. 2021, 86, 7881;
- 12cN. Mukherjee, T. Chatterjee, Green Chem. 2021, 23, 10006;
- 12dS. K. Samanta, M. K. Bera, Org. Biomol. Chem. 2019, 17, 6441;
- 12eG. Satish, A. Polu, T. Ramar, A. Ilangovan, J. Org. Chem. 2015, 80, 5167;
- 12fK. K. D. R. Viswanadham, M. P. Reddy, P. Sathyanarayana, O. Ravi, R. Kant, S. R. Bathula, Chem. Commun. 2014, 50, 13517.
- 13
- 13aW.-C. Lee, H.-C. Shen, W.-P. Hu, W.-S. Lo, C. Murali, J. K. Vandavasi, J.-J. Wang, Adv. Synth. Catal. 2012, 354, 2218;
- 13bY. Takeda, R. Kajihara, N. Kobayashi, K. Noguchi, A. Saito, Org. Lett. 2017, 19, 6744;
- 13cS. Ajarul, A. Kayet, T. Pati, D. Maiti, Chem. Commun. 2020, 56, 474;
- 13dD. P. Pace, R. Robidas, U. P. N. Tran, C. Y. Legault, T. V. Nguyen, J. Org. Chem. 2021, 86, 8154.
- 14For recent reviews on ynamide reactivity, see:
- 14aY.-C. Hu, Y. Zhao, B. Wan, Q.-A. Chen, Chem. Soc. Rev. 2021, 50, 2582;
- 14bC. C. Lynch, A. Sripada, C. Wolf, Chem. Soc. Rev. 2020, 49, 8543;
- 14cY.-B. Chen, P.-C. Qian, L.-W. Ye, Chem. Soc. Rev. 2020, 49, 8897;
- 14dF.-L. Hong, L.-W. Ye, Acc. Chem. Res. 2020, 53, 2003;
- 14eB. Zhou, T.-D. Tan, X.-Q. Zhu, M. Shang, L.-W. Ye, ACS Catal. 2019, 9, 6393.
- 15For recent examples, see:
- 15aC. Jacob, H. Baguia, A. Dubart, S. Oger, P. Thilmany, J. Beaudelot, C. Deldaele, S. Peruško, Y. Landrain, B. Michelet, S. Neale, E. Romero, C. Moucheron, V. Speybroeck, C. Theunissen, G. Evano, Nat. Commun. 2022, 13, 560;
- 15bM. Lecomte, M. Lahboubi, P. Thilmany, A. E. El Bouzakhi, G. Evano, Chem. Sci. 2021, 12, 11157;
- 15cP. Thilmany, G. Evano, Angew. Chem. Int. Ed. 2020, 59, 242; Angew. Chem. 2020, 132, 248.
- 16For recent examples, see:
- 16aS. Dutta, S. Yang, R. Vanjari, R. K. Mallick, V. Gandon, A. K. Sahoo, Angew. Chem. Int. Ed. 2020, 59, 10785; Angew. Chem. 2020, 132, 10877;
- 16bB. Prabagar, R. K. Mallick, R. Prasad, V. Gandon, A. K. Sahoo, Angew. Chem. Int. Ed. 2019, 58, 2365; Angew. Chem. 2019, 131, 2387;
- 16cS. Dutta, R. K. Mallick, R. Prasad, V. Gandon, A. K. Sahoo, Angew. Chem. Int. Ed. 2019, 58, 2289; Angew. Chem. 2019, 131, 2311.
- 17For recent examples, see:
- 17aA. M. Jadhav, V. V. Pagar, D. B. Huple, R.-S. Liu, Angew. Chem. Int. Ed. 2015, 54, 3812; Angew. Chem. 2015, 127, 3883;
- 17bS. N. Karad, R.-S. Liu, Angew. Chem. Int. Ed. 2014, 53, 9072; Angew. Chem. 2014, 126, 9218;
- 17cS. A. Gawade, D. B. Huple, R.-S. Liu, J. Am. Chem. Soc. 2014, 136, 2978.
- 18For recent examples, see:
- 18aL.-J. Qi, C.-T. Li, Z.-Q. Huang, J.-T. Jiang, X.-Q. Zhu, X. Lu, L.-W. Ye, Angew. Chem. Int. Ed. 2022, 61, e202210637; Angew. Chem. 2022, 134, e202210637;
- 18bG. Zhu, W.-C. Gao, X. Jiang, Angew. Chem. Int. Ed. 2022, 61, e202204603; Angew. Chem. 2022, 134, e202204603;
- 18cZ.-S. Wang, L.-J. Zhu, C.-T. Li, B.-Y. Liu, X. Hong, L.-W. Ye, Angew. Chem. Int. Ed. 2022, 61, e202201436; Angew. Chem. 2022, 134, e202201436;
- 18dY.-Q. Zhang, Y.-B. Chen, J.-R. Liu, S.-Q. Wu, X.-Y. Fan, Z.-X. Zhang, X. Hong, L.-W. Ye, Nat. Chem. 2021, 13, 1093;
- 18eZ.-S. Wang, Y.-B. Chen, H.-W. Zhang, Z. Sun, C. Zhu, L.-W. Ye, J. Am. Chem. Soc. 2020, 142, 3636;
- 18fF.-L. Hong, Y.-B. Chen, S.-H. Ye, G.-Y. Zhu, X.-Q. Zhu, X. Lu, R.-S. Liu, L.-W. Ye, J. Am. Chem. Soc. 2020, 142, 7618.
- 19For recent examples, see:
- 19aT. Ito, S. Harada, H. Homma, H. Takenaka, S. Hirose, T. Nemoto, J. Am. Chem. Soc. 2021, 143, 604;
- 19bG.-Y. Zhu, J.-J. Zhou, L.-G. Liu, X. Li, X.-Q. Zhu, X. Lu, J.-M. Zhou, L.-W. Ye, J. Am. Chem. Soc. 2021, 143, 1334;
- 19cL. Zeng, Y. Lin, J. Li, H. Sajiki, H. Xie, S. Cui, Nat. Commun. 2020, 11, 5639;
- 19dM. Moskowitz, C. Wolf, Angew. Chem. Int. Ed. 2019, 58, 3402; Angew. Chem. 2019, 131, 3440;
- 19eB. Huang, L. Zeng, Y. Shen, S. Cui, Angew. Chem. Int. Ed. 2017, 56, 4565; Angew. Chem. 2017, 129, 4636;
- 19fX. Tian, L. Song, M. Rudolph, F. Rominger, T. Oeser, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2019, 58, 3589; Angew. Chem. 2019, 131, 3627.
- 20
- 20aY. Zhang, R. P. Hsung, X. Zhang, J. Huang, B. W. Slafer, A. Davis, Org. Lett. 2005, 7, 1047;
- 20bN. Zheng, Y.-Y. Chang, L.-J. Zhang, J.-X. Gong, Z. Yang, Chem. Asian J. 2016, 11, 371;
- 20cY. Wang, J. Lin, X. Wang, G. Wang, X. Zhang, B. Yao, Y. Zhao, P. Yu, B. Lin, Y. Liu, M. Cheng, Chem. Eur. J. 2018, 24, 4026;
- 20dY. Wang, X. Wang, J. Lin, B. Yao, G. Wang, Y. Zhao, X. Zhang, B. Lin, Y. Liu, M. Cheng, Y. Liu, Adv. Synth. Catal. 2018, 360, 1483;
- 20eC. Liu, Z. Sun, F. Xie, G. Liang, L. Yang, Y. Li, M. Cheng, B. Lin, Y. Liu, Chem. Commun. 2019, 55, 14418;
- 20fX.-T. Lin, C. Zhao, D.-R. Wang, G.-C. Wu, G.-S. Chen, S.-J. Chen, H. Ren, D.-S. Deng, Y.-B. Xu, X.-W. Hu, Y.-L. Liu, Adv. Synth. Catal. 2022, 364, 890;
- 20gL. Li, X.-M. Chen, Z.-S. Wang, B. Zhou, X. Liu, X. Lu, L.-W. Ye, ACS Catal. 2017, 7, 4004;
- 20hY. Pang, G. Liang, F. Xie, H. Hu, C. Du, X. Zhang, M. Cheng, B. Lin, Y. Liu, Org. Biomol. Chem. 2019, 17, 2247;
- 20iG. Liang, Y. Pang, Y. Ji, K. Zhuang, L. Li, F. Xie, L. Yang, M. Cheng, B. Lin, Y. Liu, J. Org. Chem. 2020, 85, 3010;
- 20jY. Chen, Z. Wang, W. Zhao, S. Sun, L. Yang, J. Zhang, D. Zhang, M. Cheng, B. Lin, Y. Liu, Chem. Commun. 2022, 58, 3051.
- 21
- 21aA. Suμrez, S. Suμrez-Pantiga, O. Nieto-Faza, R. Sanz, Org. Lett. 2017, 19, 5074;
- 21bF. Martínez-Lara, A. Suárez, S. Suárez-Pantiga, M. J. Tapia, R. Sanz, Org. Chem. Front. 2020, 7, 1869;
- 21cA. S. K. Hashmi, W. Yang, F. Rominger, Chem. Eur. J. 2012, 18, 6576;
- 21dZ. Zhang, X. Tang, Q. Xu, M. Shi, Chem. Eur. J. 2013, 19, 10625;
- 21eS. Yaragorla, D. Bag, R. Dada, K. Jose, ACS Omega 2018, 3, 15024.
- 22For selected examples on I2-promoted diindolyl-alkynes cycloisoemrization, see:
- 22aK. Win, A. Sonawane, M. Koketsu, Org. Biomol. Chem. 2021, 19, 3199;
- 22bS. D. Gawande, V. Kavala, M. R. Zanwar, C.-W. Kuo, H.-N. Huang, C.-H. He, T.-S. Kuo, C.-F. Yao, Adv. Synth. Catal. 2013, 355, 3022.
- 23
- 23aW.-T. Guo, B.-H. Zhu, Y. Chen, J. Yang, P.-C. Qian, C. Deng, L.-W. Ye, L. Li, J. Am. Chem. Soc. 2022, 144, 6981;
- 23bW.-F. Luo, L.-W. Ye, L. Li, P.-C. Qian, Chem. Commun. 2021, 57, 5032;
- 23cB.-H. Zhu, C.-H. Shen, M.-L. Nie, F. Zheng, C. Huang, F. Chen, L. Li, C. Deng, L.-W. Ye, P.-C. Qian, Org. Lett. 2022, 24, 7009–7014.
- 24Deposition Numbers 2206855 (3), 2206856 (for 10), 2206853 (for 43) and 2206852 (for 63) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 25
- 25aM. T. Robak, M. A. Herbage, J. A. Ellman, Chem. Rev. 2010, 110, 3600;
- 25bJ. A. Ellman, Pure Appl. Chem. 2003, 75, 39;
- 25cJ. A. Ellman, T. D. Owens, T. P. Tang, Acc. Chem. Res. 2002, 35, 984.
- 26For selected examples on the indole rearrangement, see:
- 26aC. Zheng, S.-L. You, Acc. Chem. Res. 2020, 53, 974;
- 26bC. Zheng, Z.-L. Xia, S.-L. You, Chem 2018, 4, 1952;
- 26cY. Wang, C. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2017, 56, 15093; Angew. Chem. 2017, 129, 15289;
- 26dC.-X. Zhuo, Q.-F. Wu, Q. Zhao, Q.-L. Xu, S.-L. You, J. Am. Chem. Soc. 2013, 135, 8169.