Low-Valent MxAl3 Cluster Salts with Tetrahedral [SiAl3]+ and Trigonal-Bipyramidal [M2Al3]2+ Cores (M=Si/Ge)
Graphical Abstract
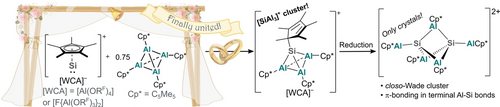
The elusive tetrahedral [Cp*Si(AlCp*)3]+ cation is prepared as its aluminate salts starting from Jutzi's [SiCp*]+ cation and Schnöckel's [(AlCp*)4]. Owing to its good accessibility, [SiAl3]+ can act as starting point towards the synthesis of low-valent silicon-doped aluminium-cluster cores as shown by the preparation of the larger [Si2(AlCp*)5]2+ cluster cation.
Abstract
Schnöckel's [(AlCp*)4] and Jutzi's [SiCp*][B(C6F5)4] (Cp*=C5Me5) are landmarks in modern main-group chemistry with diverse applications in synthesis and catalysis. Despite the isoelectronic relationship between the AlCp* and the [SiCp*]+ fragments, their mutual reactivity is hitherto unknown. Here, we report on their reaction giving the complex salts [Cp*Si(AlCp*)3][WCA] ([WCA]−=[Al(ORF)4]− and [F{Al(ORF)3}2]−; RF=C(CF3)3). The tetrahedral [SiAl3]+ core not only represents a rare example of a low-valent silicon-doped aluminium-cluster, but also—due to its facile accessibility and high stability—provides a convenient preparative entry towards low-valent Si−Al clusters in general. For example, an elusive binuclear [Si2(AlCp*)5]2+ with extremely short Al−Si bonds and a high negative partial charge at the Si atoms was structurally characterised and its bonding situation analysed by DFT. Crystals of the isostructural [Ge2(AlCp*)5]2+ dication were also obtained and represent the first mixed Al−Ge cluster.
Introduction
Molecular silicon clusters have been heavily investigated due to promising applications ranging from small-molecule activation to the deposition of silicon as bulk and thin films.1 Synthetic chemists have developed a library of structurally-diverse neutral and anionic, saturated and unsaturated silicon clusters. For example, saturated tetrasilatetrahedranes,2 hexasilaprismanes,3 and octasilacubanes4 have been isolated, but also the unsaturated dismutational isomer of hexasilabenzene as well as the corresponding global minimum isomer.5, 6 The anionic congeners7-9 constitute valuable starting materials for the incorporation of group 13 (B) and 15 (P) atoms into the silicon scaffold.8 The atomically precise expansion of siliconoids10 as well as germanium-substituted derivatives has been reported more recently.11-13 In contrast, mixed cluster compounds with the similarly abundant metal aluminium, for which an extensive cluster chemistry has been developed separately,14 are mostly restricted to the endohedral incorporation of silicon atoms. Jutzi and Schnöckel reported the synthesis of the unique Si@Al14Cp*6 cluster containing a Si@Al8 core (I),15 which has since than also been observed in the analogous Si@Al14(N(Dipp)SiMe3)6 cluster (Dipp=2,6-iPr2C6H3)16 and, although distorted, in the superatomic Si@Al56[N(Dipp)SiMe3]12 cluster (Scheme 1).17 Other than these, only saturated Si−Al compounds including SiAl3 (II) and SiAl4 (IV) cores were hitherto reported.18 The trigonal bipyramidal closo-Al4Si cluster (V) synthesised by Schnöckel is the sole example with a silicon atom incorporated into the polyhedral aluminium scaffold.19
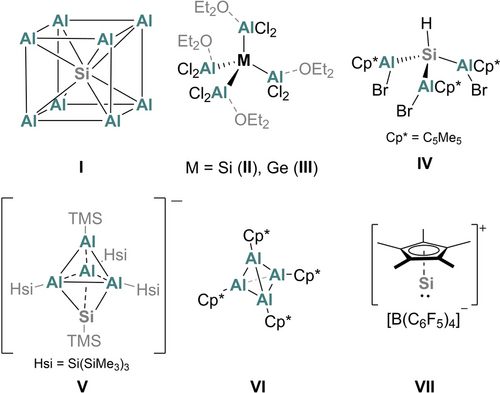
Central Si@Al8 unit discovered in large mixed clusters, literature known mixed low-valent Si−Al compounds as well as Schnöckel's [(AlCp*)4] and Jutzi's [SiCp*]+. Hsi=Si(SiMe3)3; TMS=SiMe3; Cp*=C5Me5.
Although these structures I–V already hint to the diversity of assemblies possible in low-valent Si−Al clusters, a more detailed analysis of the bonding and especially follow-up chemistry is limited by the need for metastable AlX (X=Cl, Br, I) solutions as Al source in the synthesis of the above presented species. AlX solutions are typically prepared in situ in a highly specialised apparatus.20 Due to the high reactivity of aluminium(I) halides, the products are often only prepared in low yields.
Hence, we were interested to synthesise low-valent Si−Al species in a systematic manner from accessible starting materials. Schnöckel's tetrahedral [(AlCp*)4]21 (VI, Cp*=C5Me5) and Jutzi's [SiCp*][B(C6F5)4]22 (VII) are among the most prominent protagonists of low-valent main-group chemistry. Moreover, both molecules became more readily available in recent years23 and are widely applied starting materials for low-valent Al and Si chemistry.24, 25 Yet, the mutual reaction between VI and VII has never been reported.
Here, we describe the synthesis and characterisation of complex salts with tetrahedral [SiAl3]+ cluster core stabilised by weakly-coordinating anions (WCAs) that were obtained from the reaction of [SiCp*][WCA] with [(AlCp*)4] ([WCA]−=[Al(ORF)4]− and [F{Al(ORF)3}2]−; RF=C(CF3)3). Moreover, the formation of larger clusters featuring the dications [M2(AlCp*)5]2+ (M=Si, Ge) serves as proof-of-principle demonstrating the potential to access larger mixed group 13/14 clusters starting from [SiAl3]+ precursors.
Results and Discussion
Syntheses and Basic Characterisation
The [Al(ORF)4]− salt of Jutzi's silicocenium cation, [SiCp*][Al(ORF)4] 1 A, was synthesised in 88 % yield in analogy to a known procedure via the reaction of [H(Et2O)2][Al(ORF)4] with SiCp*2 (Scheme 2a).26 The complex salt [SiCp*][F{Al(ORF)3}2] 1 B with the even less coordinating [F{Al(ORF)3}2]− anion was synthesised by abstraction of a Cp* ligand from SiCp*2 with the masked silylium agent Me3Si−F−Al(ORF)3.27 Addition of two equivalents of Me3Si−F−Al(ORF)3 directly yielded the fluoride-bridged anion in 1 B with 87 % yield (Scheme 2b). The aluminate salts of the heavier germanocenium cation in [GeCp*][WCA] ([WCA]−=[Al(ORF)4]− (2 A), [F{Al(ORF)3}2]− (2 B)) were synthesised by the analogous reaction routes (2 A 88 % yield, 2 B 73 % yield, Scheme 2a/b). The complex salts were obtained as scXRD-quality crystals.28 The bulk purity was confirmed NMR spectroscopically (Supporting Information, section 1). The compounds are stable at rt in the solid state and in 1,2-difluorobenzene (1,2-DFB) solution.
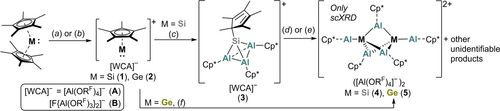
Synthesis of novel aluminate salts of [MCp*]+ (M=Si, Ge) and formation of M−Al clusters upon addition of [(AlCp*)4]. Reaction conditions: a) [H(Et2O)2][Al(ORF)4], −Cp*H, PhF, −40 °C, 3 h; b) 2 Me3SiF−Al(ORF)3, −Me3SiF, −Cp*SiMe3, 1,2-DFB, rt, 3 h; c)  [(AlCp*)4], 1,2-DFB, −40 °C, 2 h; d) 1,2-DFB, 60 °C, 10 h, −[AlCp*2][Al(ORF)4]; (e) [Mg(NacNacmes)]2, 1,2-DFB, rt, 5 min; f)
[(AlCp*)4], 1,2-DFB, −40 °C, 2 h; d) 1,2-DFB, 60 °C, 10 h, −[AlCp*2][Al(ORF)4]; (e) [Mg(NacNacmes)]2, 1,2-DFB, rt, 5 min; f)  [(AlCp*)4], −[AlCp*2][Al(ORF)4], 1,2-DFB, rt, 5 min ; NacNacMes=[(MesNCMe)2CH]− with Mes=2,4,6-(Me)3C6H3.
[(AlCp*)4], −[AlCp*2][Al(ORF)4], 1,2-DFB, rt, 5 min ; NacNacMes=[(MesNCMe)2CH]− with Mes=2,4,6-(Me)3C6H3.
Mixed M-Al Clusters
Aiming for the isolation of a mixed Si−Al cluster, the silicocenium salts were each reacted with 0.75 equiv of [(AlCp*)4] at −40 °C in 1,2-DFB. After 2 h, the formation of an orange solution was observed in both cases from which orange crystals were isolated upon crystallisation induced by layering the solution with n-heptane. scXRD analysis revealed the successful synthesis of the tetrahedral cluster cations [Cp*Si(AlCp*)3]+ as complex salts with [Al(ORF)4]− (3 A, 59 % yield) or [F{Al(ORF)3}2]− counterions (3 B 77 % yield; Scheme 2c).28
Heating a solution of 3 A in 1,2-DFB for 10 h to 60 °C resulted in partial decomposition of the cluster. NMR spectroscopy revealed the formation of [AlIIICp*2]+, which suggests that AlICp* disproportionates and acted as a reducing agent. Various other products were detected in the 1H NMR spectrum (Supporting Information, Figure S32), but unfortunately not identified. Nonetheless, crystallisation yielded a few yellow crystals of the dicationic cluster [Si2(AlCp*)5]([Al(ORF)4])2 (4)28 next to yellow powder and colourless crystals of [AlCp*2][Al(ORF)4] (Scheme 2d). Similarly, a few crystals of 4 were obtained by the reaction of 3 A with Jones's [Mg(NacNacMes)]2 (NacNacMes=[(MesNCMe)2CH]− with Mes=2,4,6-(Me)3C6H3),29 albeit next to various unidentifiable side products. In an analogous manner, yellow crystals of the germanium-analogue [Ge2(AlCp*)5]([Al(ORF)4])2 (5) were isolated from the reaction of 2 A with  [(AlCp*)4] at −40 °C,28 which represents the first ever structurally characterised mixed Ge−Al cluster. In this case, the [GeAl3]+ tetrahedron, the plausible intermediate, could not be observed even at −40 °C. Similar to the synthesis of the silicon analogue 4, the formation of [AlIII(Cp*)2]+ was confirmed by NMR spectroscopy (Scheme 2f).
[(AlCp*)4] at −40 °C,28 which represents the first ever structurally characterised mixed Ge−Al cluster. In this case, the [GeAl3]+ tetrahedron, the plausible intermediate, could not be observed even at −40 °C. Similar to the synthesis of the silicon analogue 4, the formation of [AlIII(Cp*)2]+ was confirmed by NMR spectroscopy (Scheme 2f).
Molecular Structure and Spectroscopic Characterisation of the SiAl3+ Clusters
In the molecular structure of 3 A, a silyliumylidene-type [Si(η1-Cp*)]+ cation has formally substituted an AlCp* unit in the starting material [(AlCp*)4] (Figure 1a). Analysis of the structure of the cation in 3 A reveals barely significant elongation of the Al−Al bonds compared to Schnöckel's [(AlCp*)4] tetrahedron (d(Al−Al)avg.=2.769)21 with values ranging from 2.769(2) Å to 2.816(2) Å (d(Al−Al)avg.=2.798 Å). Moreover, the observed Si−C (d(Si−C)=1.954(4) Å/1.964(4) Å) and Si−Al bond lengths (d(Si−Al)range=2.465(2)–2.522(2) Å, d(Si−Al)avg.=2.485 Å) fit well to values observed for Si−C and Si−Al single bonds (cf. d((TMS)Si−Al)=2.445(2) Å) in V).19 The tetrahedron is compressed with larger Al−Si−Al angles ranging from 67.73(4)° to 69.42(4)° (∡(Al−Si−Al)avg.=68.50) compared to Al−Al−Al angles (∡(Al−Al−Al)range=59.95(4)° to 60.64(4)°, ∡(Al−Al−Al)avg.=60.00°). Unfortunately, extensive disorder of the anions and cations in the molecular structure of 3 B precludes a detailed comparison of the bonding parameters. Nevertheless, the connectivity of the [SiAl3]+ cluster can unambiguously be identified as the same as in 3 A.
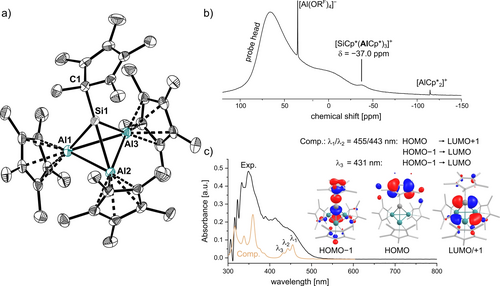
a) Molecular structure of the [Cp*Si(AlCp*)3]+ cation in 3 A. Hydrogen atoms and [Al(ORF)4]− anions omitted for clarity. Thermal displacement of the ellipsoids was set at 50 % probability. b) 27Al NMR spectrum (104.3 MHz, 1,2-DFB, 298 K) of 3 A. c) Experimental UV/Vis spectrum (1,2-DFB, rt) of 3 A compared to the theoretical UV/Vis spectrum of [Cp*Si(AlCp*)3]+ computed with TD-DFT at BP86-D3BJ/def2-SVP level of DFT. Moreover, molecular orbitals representing the ground states of the electronic transitions are displayed (isovalue 0.04).
NMR Spectra
The bulk purity of the products was confirmed by NMR and vibrational spectroscopy. In the 1H NMR spectrum of 3 A/3 B, sharp singlets for the protons of the AlCp* and [SiCp*]+ units are observed at 1.70 and 1.85 ppm. This observation points to a fluxional behaviour of the η1-coordinated Cp* ligand at silicon, but suggests that any exchange of Cp* ligands between silicon and the aluminium centres is slow on the NMR time scale.30 The 27Al NMR resonance of the cationic part of 3 A/3 B at −37.0 ppm is more low-field shifted compared to those of the related [M(AlCp*)3]+ (M=Al, Ga, In, Tl)25, 31 as well as to [(AlCp*)4] (Figure 1b). Moreover, the silicon atom gives rise to a signal at δ29Si=+57.9 ppm (cf. 1: δ29Si=−397.4 ppm).
Raman Spectra
For compounds 3 A/3 B, bands assigned to the stretching vibrations of the Si−Al bonds in the SiAl3-cluster, the tetrahedral breathing modes, were detected at 454 cm−1 and 463/461 cm−1 (see Supporting Information, Figure S29). These values compare well to M−Al stretching vibrations reported for the cationic complexes of type [M(AlCp*)3]+ (M=Al,25 Ga-Tl31) but are higher compared to the Raman band of [(AlCp*)4] ( =378 cm−1). This indicates a higher strength of the M−Al bonds in the intermetallic compounds compared to the Al−Al bonds in tetrameric [(AlCp*)4].
=378 cm−1). This indicates a higher strength of the M−Al bonds in the intermetallic compounds compared to the Al−Al bonds in tetrameric [(AlCp*)4].
UV/Vis-Data
In accordance with the orange colour of 3 A, the UV/Vis spectrum of 3 A shows a broad shoulder between 400 and 500 nm (Figure 1c). In contrast, the broad UV/Vis bands of yellow [(AlCp*)4] in 1,2-DFB do not absorb with significant intensity above 400 nm. TD-DFT computations assign the broad shoulder to electronic transitions from the HOMO centred at the η1-Cp* ligand and HOMO-1 with lone-pair character at Si into the degenerate LUMO/+1 with px/py character at Si.
Bonding within the Tetrahedral [SiAl3]+ Cluster
EDA-NOCV-Analyses
Insight into the differences of the bonding interactions within the new and known tetrahedral clusters were gained by EDA-NOCV calculations (energy decomposition analysis with natural orbitals for chemical valence). Here, interactions of the aluminylene and silyliumylidene fragments with the (AlCp*)3 trimer were studied (Figure 2a). In [(AlCp*)4], the EDA-NOCV yields an interaction energy of ΔEInt=−61.2 kcal mol−1 with an orbital contribution of ΔEOrb=−95.5 kcal mol−1. As visualised by the plots of the deformation densities, the orbital interaction is equally composed of a delocalisation of the AlCp* lone-pair orbital into the LUMO of the (AlCp*)3 fragment and the back donation of electron density from occupied ligand-group orbitals of (AlCp*)3 into empty px/py-orbitals at AlCp* (Figure 2b). The interaction energies, however, are more than doubled in the [Cp*Si(AlCp*)3]+ cluster (ΔEInt=−143.1 kcal mol−1, ΔEOrb=−222.7 kcal mol−1). In addition, the back donation of electron density from the (AlCp*)3 fragment into empty p orbitals at the silyliumylidene-type cation has become the major interaction, while the delocalisation of the lone pair at silicon represents only a minor interaction (Figure 2c). Overall, the orbital interaction becomes the dominating contribution to the total attractive interaction energy in the [SiAl3]+ cluster (60 %). In [(AlCp*)4], the orbital interaction term contributes with only 41 % to the attractive interaction energy, with the rest representing dispersion forces (12 %) and electrostatic interactions (47 %).
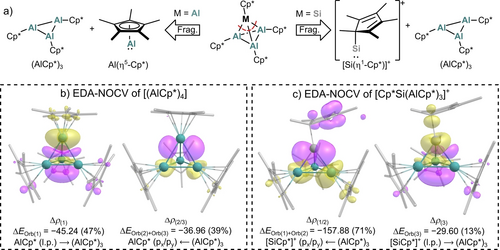
a) Fragmentation (Frag.) of the [(AlCp*)4] and [Cp*Si(AlCp*)3]+ tetrahedra in the EDA-NOCV analysis into singlet (S) fragments. b) Plots of deformation density Δρ (isovalue 0.002) associated with the major contributing energy terms ΔEOrb (values in kcal mol−1) for fragmentation of [(AlCp*)4] into AlCp* (S) and (AlCp*)3 (S). c) Plots of deformation density Δρ (isovalue 0.002) associated with the major contributing energy terms ΔEOrb (values in kcal mol−1) for fragmentation of [Cp*Si(AlCp*)3]+ into [Si(η1-Cp*)]+ (S) and (AlCp*)3 (S). Charge flows from yellow to purple.
A similar bonding interaction compared to [Cp*Si(AlCp*)3]+ was computed for the recently reported [Al(AlCp*)3]+ cation,25 although the interaction energies of the Al+ cation with the (AlCp*)3 unit in [Al(AlCp*)3]+ are considerably smaller compared to those in [Cp*Si(AlCp*)3]+.
QTAIM-Analyses
The high bond strength of the Si−Al bonds is also reflected in the QTAIM analysis (quantum theory of atoms in molecules). Here, the electron density ρr at the BCPs (bond critical point) of the Si−Al bonds averages to a value of 0.35 e− Å−3 (cf. Al−Al in [(AlCp*)4]: ρr, avg.=0.29 e− Å−3). In accordance with the scXRD analysis, the lower electron densities at the BCPs of the Al−Al bonds in [SiAl3]+ (ρr, avg.=0.23 e− Å−3) confirm the weakening of the Al−Al bonds. Intriguingly, the computed QTAIM charge at the silicon atom is significantly negative (qSi=−0.52) while high positive charges are calculated for the Al atoms (qAl, avg.=+1.35; qAlCp*, avg.=−0.67; cf. [(AlCp*)4]: qAl, avg.=0.84). Hence, a formal reduction of the silicon atom has occurred.
Molecular Structures with Trigonal Bipyramidal M2Al3 Core (M=Si, Ge)
Main-group metal-clusters with trigonal bipyramidal frameworks have been widely reported in group 14 in the homonuclear, heavier [1.1.1]propellane analogues.32, 33 Moreover, heteronuclear Ge2Sn3 and Ga2Ge3 cluster cores with formally bridging tetrylenes are known.34 For group 13 metals, the structural motif can be found in clusters with alkali metals of type M12[(M2R)3] (M1/M2=Na/Al,35 Na/Ga,36 K/Ga;37 R=Mes2C6H3), representing an aromatic [M3]2− ring bicapped electrostatically by alkali-metal cations. Notably, similar structural motifs as the central [M2(AlCp*)3] cluster core in 4 and 5 were reported for the only other known main-group metal-AlCp* clusters (M=As,38 Sb, Bi39). In the cluster cores of 4 and 5, two group 14 metal atoms are connected by three bridging AlCp* units (Figure 3). Two AlCp* moieties are coordinated end-on at Si/Ge and cap the complex. The Si−Al bonds in the Si2Al3 core (d(Si−Albrid.)avg.=2.467 Å) are of similar length compared to Si−Al bonds in the tetrahedron 3. Yet, the terminal Si−Al bonds are significantly shortened to 2.327(1) Å and represent to the best of our knowledge the shortest structurally characterised Si−Al bonds.
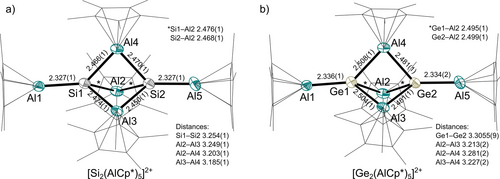
a) Molecular structure of the [Si2(AlCp*)5]2+ cation in 4. Hydrogen atoms and [Al(ORF)4]− anions omitted for clarity. Thermal displacement of the ellipsoids was set at 50 % probability. b) Molecular structure of the [Ge2(AlCp*)5]2+ cation in 5. Hydrogen atoms and [Al(ORF)4]− anions omitted for clarity. Thermal displacement of the ellipsoids was set at 50 % probability.
Similar differences of the bond lengths are observed in the Ge2Al5 cluster with short terminal Ge−Al bonds at 2.336(1)/2.334(2) Å. The Si−Si/Ge−Ge distances of 3.254(1) Å and 3.3055(9) Å are much longer than the corresponding distances between the naked Si/Ge atoms in the aforementioned benzpolarene and propellane motifs (2.55–2.78 Å),5-12, 33 which is in line with the expected absence of a bonding interaction in 4 and 5.
Bonding within the Trigonal Bipyramidal M2Al3 Clusters (M=Si, Ge)
Just as the HOMO in the reported [M2(AlCp*)3] (M=As,38 Sb, Bi39) clusters, the degenerate HOMO/-1 in 4 and 5 display Si/Ge-centered p orbitals (Figure 4a). Analysis of the molecular orbitals reveals the bonding in the M2Al3 cluster core to be best described as closo-Wade cluster. The six skeletal electron pairs (SEP) of the trigonal-bipyramidal Si2Al3 cluster (Figure 4b) match the SEPs computed for the model compound closo-[B5H5]2− (Supporting Information, Figure S46). However, the long Al−Al distances and the absence of BCPs in the QTAIM analysis exclude a strong covalent interaction between adjacent bridging Al atoms. SEPs and high electron density on ring critical points (RCPs) in Si2Al3 imply weakly bonding Al−Al interactions as expected for a closo-cluster (Figure 4c).
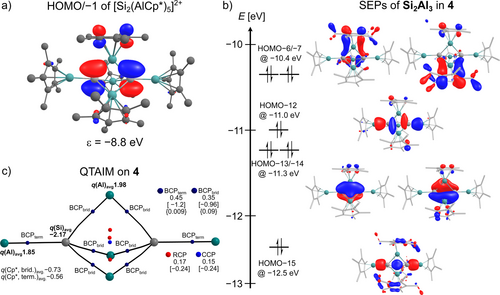
a) HOMO/−1 of closo-[Si2(AlCp*)5]2+ computed at the BP86-D3BJ/def2-SVP level of DFT (isovalue 0.04). b) Bonding skeletal orbitals SEPs (isovalue 0.03) of closo-[Si2(AlCp*)5]2+ computed at the BP86-D3BJ/def2-SVP level of DFT. c) Results of the QTAIM analysis for [Si2(AlCp*)5]2+ with average values for electron density ρr in e− Å−3 [Laplacian of electron density Δρr in e− Å−5] {bond ellipticity ϵ} at the critical points (BCP=bond critical points, RCP=ring critical point, CCP=cluster critical points).
The high electron densities residing on the BCPs of terminal Al−Si bonds (ρr=0.45 e− Å−3) are consistent with the strong bonding interactions suggested by the short bond lengths. The low values for the bond ellipticities of ϵ<0.01 at the BCPs of terminal Al−Si bonds indicate no anisotropy of the curvature of the electron density orthogonal to the bond, as found for single or triple bonds.40 Moreover, even larger negative QTAIM charges at the Si atoms in 4 are computed as compared to the Si atom in 3, while the Al atoms bear higher positive charges. Similarly, negative QTAIM charges are computed for the Ge atoms in 5 (Supporting Information, Figure S57).
EDA-NOCV-Analyses
To further investigate the bonding situation in 4, the Si−Al bonds were studied by EDA-NOCV analyses (Figure 5; Supporting Information, section 2). For the bridging AlCp* units, only minimal differences in energy are computed for a heterolytic fragmentation into singlet or the alternative homolytic fragmentation into triplet fragments (heterolytic: ΔEOrb(1)=−244.57 kcal mol−1, homolytic: ΔEOrb(1)=−238.04 kcal mol−1, see Figure 5a and Table S5). For simplification, only the fragmentation into singlet fragments is discussed. Here, the donation of electron density from the lone pair at the AlCp* moiety into p orbitals at the silicon atoms represents the major orbital interaction. This observation fits the highly positive QTAIM charges at the bridging Al atoms as well as the computed HOMO/-1 of the complex.
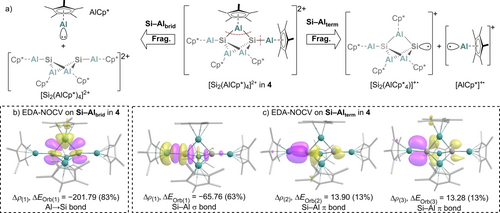
a) Fragmentation (Frag.) of the Si−Al bonds of the bridging and the terminal AlCp* units in 4 in the EDA-NOCV analysis into fragments. b) Plots of deformation density Δρ (isovalue 0.003) associated with the major contributing energy term ΔEOrb (values in kcal mol−1) for fragmentation of bridging Si−Al−Si bonds in AlCp* (S) and [Si2(AlCp*)4]2+ (S) fragments. c) Plots of deformation density Δρ (isovalue 0.001) associated with the major contributing energy terms ΔEOrb (values in kcal mol−1) for fragmentation of terminal Al−Si bond in [AlCp*]+ (D) and [Si2(AlCp*)4]+(D) fragments. Charge flows from yellow to purple.
Moreover, the exceptionally short terminal Al−Si bond lengths at only 2.327(1) Å (Si) and 2.335(2) Å (Ge) can be rationalised by EDA-NOCV analyses. Here, the homoleptic fragmentation into two doublet fragments is computed to be more meaningful (Figure 5a; Supporting Information, Table S4). In addition to the σ bonding interaction (Δρ(1) in Figure 5c), delocalisation of electron density from the M2Al3 core and p orbitals of the other Si atom to the Al−Si bond results in two π-bonding interactions (Δρ(2) and Δρ(3) in Figure 5c). These results are supported by the low bond ellipticity of ϵ=0.009 that results from the two almost equal, but orthogonal π-interactions and agrees with the high electron densities residing on the terminal Si−Al BCPs as calculated in the QTAIM analysis in Figure 4c.
Conclusion
The rational synthesis of the tetrahedral [Cp*Si(AlCp*)3]+ cluster stabilised with the weakly coordinating anions [Al(ORF)4]− and [F{Al(ORF)3}2]− is reported. The cluster was obtained by reaction of the readily available corresponding [SiCp*]+ salts with [(AlCp*)4] in 1,2-difluorobenzene. EDA-NOCV and QTAIM analyses revealed a significantly higher stability of the cationic SiAl3+ cluster compared to the iconic neutral Al4 cluster in [(AlCp*)4]. Yet, the cluster appears to be only metastable, as the silicon atom in [SiAl3]+ bears a negative QTAIM charge and formation of the larger cluster [Si2(AlCp*)5]2+ was observed upon heating, which is accompanied by further a reduction of the silicon atoms. In contrast, the homologous mixed [GeAl3]+ tetrahedron appears to be unstable towards disproportionation into larger, unidentifiable clusters of which the salt including the [Ge2(AlCp*)5]2+ dication could be structurally characterised. Future research will focus on expanding the field of mixed Si−Al and Si−Ge clusters and exploring the follow-up chemistry of the straightforwardly accessible cationic [SiAl3]+ tetrahedron.
Acknowledgments
We thank the Fonds of the Chemical Industry FCI for a fellowship for Philipp Dabringhaus, the German Research Foundation (DFG) for the funding of projects KR2046/35-1 and SCHE906/4-4 as well as the Albert-Ludwigs-University Freiburg and Saarland University for supporting the work. Furthermore, we thank Manuel Schmitt for help with the quantum-chemical calculations. We acknowledge Harald Scherer and Fadime Bitgül for measurement of NMR spectra. Furthermore, the authors acknowledge support by the state of Baden-Württemberg through bwHPC and the DFG through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster). Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
There are no conflicts to declare.
Open Research
Data Availability Statement
X-ray crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under the reference numbers CCDC 2209238 (1 A), 2209239 (1 B), 2210642 (2 A), 2209240 (2 B), 2209241 (3 A), 2210870 (3 B), 2210446 (4), and 2210639 (5). All other data supporting the findings are contained in the main text or the Supporting Information.