Planar Hexacoordinate Carbons: Half Covalent, Half Ionic
Luis Leyva-Parra
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Search for more papers by this authorLuz Diego
Escuela Profesional de Química, Facultad de Ciencias Naturales, Universidad Nacional Federico Villarreal, Jr. Río Chepén 290, El Agustino, Lima, Perú
Search for more papers by this authorDr. Osvaldo Yañez
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Center of New Drugs for Hypertension (CENDHY), Santiago, Chile
Search for more papers by this authorDiego Inostroza
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Search for more papers by this authorCorresponding Author
Jorge Barroso
Departamento de Física Aplicada, Centro de Investigación y de Estudios Avanzados, Unidad Mérida, km. 6 Antigua carretera a Progreso, Apdo. Postal 73, Cordemex Mérida, Yuc., México
Search for more papers by this authorCorresponding Author
Dr. Alejandro Vásquez-Espinal
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Search for more papers by this authorCorresponding Author
Prof. Gabriel Merino
Departamento de Física Aplicada, Centro de Investigación y de Estudios Avanzados, Unidad Mérida, km. 6 Antigua carretera a Progreso, Apdo. Postal 73, Cordemex Mérida, Yuc., México
Search for more papers by this authorCorresponding Author
Prof. William Tiznado
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Search for more papers by this authorLuis Leyva-Parra
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Search for more papers by this authorLuz Diego
Escuela Profesional de Química, Facultad de Ciencias Naturales, Universidad Nacional Federico Villarreal, Jr. Río Chepén 290, El Agustino, Lima, Perú
Search for more papers by this authorDr. Osvaldo Yañez
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Center of New Drugs for Hypertension (CENDHY), Santiago, Chile
Search for more papers by this authorDiego Inostroza
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Search for more papers by this authorCorresponding Author
Jorge Barroso
Departamento de Física Aplicada, Centro de Investigación y de Estudios Avanzados, Unidad Mérida, km. 6 Antigua carretera a Progreso, Apdo. Postal 73, Cordemex Mérida, Yuc., México
Search for more papers by this authorCorresponding Author
Dr. Alejandro Vásquez-Espinal
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Search for more papers by this authorCorresponding Author
Prof. Gabriel Merino
Departamento de Física Aplicada, Centro de Investigación y de Estudios Avanzados, Unidad Mérida, km. 6 Antigua carretera a Progreso, Apdo. Postal 73, Cordemex Mérida, Yuc., México
Search for more papers by this authorCorresponding Author
Prof. William Tiznado
Computational and Theoretical Chemistry Group, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, República 498, Santiago, Chile
Search for more papers by this authorGraphical Abstract
Abstract
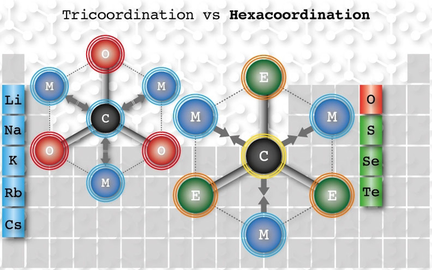
Herein, the first global minima containing a planar hexacoordinate carbon (phC) atom are reported. The fifteen structures belong to the CE3M3+ (E=S–Te and M=Li–Cs) series and satisfy both geometric and electronic criteria to be considered as a true phC. The design strategy consisted of replacing oxygen in the D3h CO3Li3+ structure with heavy and less electronegative chalcogens, inducing a negative charge on the C atom and an attractive electrostatic interaction between C and the alkali-metal cations. The chemical bonding analyses indicate that carbon is covalently bonded to three chalcogens and ionically connected to the three alkali metals.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202100940-sup-0001-misc_information.pdf8.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Hoffmann, R. W. Alder, C. F. Wilcox, J. Am. Chem. Soc. 1970, 92, 4992–4993.
- 2J. B. Collins, J. D. Dill, E. D. Jemmis, Y. Apeloig, P. v. R. Schleyer, R. Seeger, J. A. Pople, J. Am. Chem. Soc. 1976, 98, 5419–5427.
- 3F. A. Cotton, M. Millar, J. Am. Chem. Soc. 1977, 99, 7886–7891.
- 4R. Keese, A. Pfenninger, A. Roesle, Helv. Chim. Acta 1979, 62, 326–334.
- 5Z.-X. Wang, P. v. R. Schleyer, Science 2001, 292, 2465–2469.
- 6V. Vassilev-Galindo, S. Pan, K. J. Donald, G. Merino, Nat. Rev. Chem. 2018, 2, 114.
- 7R. Grande-Aztatzi, J. L. Cabellos, R. Islas, I. Infante, J. M. Mercero, A. Restrepo, G. Merino, Phys. Chem. Chem. Phys. 2015, 17, 4620–4624.
- 8Y. Pei, W. An, K. Ito, P. v. R. Schleyer, X. C. Zeng, J. Am. Chem. Soc. 2008, 130, 10394–10400.
- 9Y. Wang, F. Li, Y. Li, Z. Chen, Nat. Commun. 2016, 7, 11488.
- 10Z.-h. Cui, V. Vassilev-Galindo, J. L. Cabellos, E. Osorio, M. Orozco, S. Pan, Y.-h. Ding, G. Merino, Chem. Commun. 2017, 53, 138–141.
- 11S. Pan, J. L. Cabellos, M. Orozco-Ic, P. K. Chattaraj, L. Zhao, G. Merino, Phys. Chem. Chem. Phys. 2018, 20, 12350–12355.
- 12J.-C. Guo, L.-Y. Feng, J. Barroso, G. Merino, H.-J. Zhai, Chem. Commun. 2020, 56, 8305–8308.
- 13Y. Li, Y. Liao, Z. Chen, Angew. Chem. Int. Ed. 2014, 53, 7248–7252; Angew. Chem. 2014, 126, 7376–7380.
- 14K. Exner, P. v. R. Schleyer, Science 2000, 290, 1937–1940.
- 15B. B. Averkiev, D. Y. Zubarev, L. M. Wang, W. Huang, L.-S. Wang, A. I. Boldyrev, J. Am. Chem. Soc. 2008, 130, 9248–9250.
- 16Y.-B. Wu, Y. Duan, G. Lu, H.-G. Lu, P. Yang, P. v. R. Schleyer, G. Merino, R. Islas, Z.-X. Wang, Phys. Chem. Chem. Phys. 2012, 14, 14760–14763.
- 17W. N. Setzer, P. v. R. Schleyer, Adv. Organomet. Chem. 1985, 24, 353–451.
- 18P. Muller, Pure Appl. Chem. 1994, 66, 1077–1184.
- 19A. M. Pendás, M. A. Blanco, E. Francisco, J. Chem. Phys. 2004, 120, 4581–4592.
- 20A. M. Pendás, E. Francisco, M. A. Blanco, J. Comput. Chem. 2005, 26, 344–351.
- 21M. A. Blanco, A. M. Pendás, E. Francisco, J. Chem. Theory Comput. 2005, 1, 1096–1109.
- 22A. M. Pendás, M. A. Blanco, E. Francisco, J. Comput. Chem. 2007, 28, 161–184.
- 23S. M. Aucott, C. J. Burchell, A. M. Z. Slawin, J. D. Woollins, Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 903–906.
- 24C. J. Burchell, S. M. Aucott, A. M. Z. Slawin, J. D. Woollins, Dalton Trans. 2005, 4, 735–739.
- 25M. Kazemi, L. Shiri, H. Kohzadi, Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 1398–1409.
- 26O. Yañez, R. Báez-Grez, D. Inostroza, W. A. Rabanal-León, R. Pino Rios, J. Garza, W. Tiznado, J. Chem. Theory Comput. 2019, 15, 1463–1475.
- 27R. Grande-Aztatzi, P. R. Martínez-Alanis, J. L. Cabellos, E. Osorio, A. Martínez, G. Merino, J. Comput. Chem. 2014, 35, 2288–2296.
- 28C. Adamo, V. Barone, J. Chem. Phys. 1999, 110, 6158–6170.
- 29S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104.
- 30P. Fuentealba, L. Von Szentpaly, H. Preuss, H. Stoll, J. Phys. B 1985, 18, 1287.
- 31A. Bergner, M. Dolg, W. Küchle, H. Stoll, H. Preuss, Mol. Phys. 1993, 80, 1431–1441.
- 32F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 2005, 7, 3297–3305.
- 33R. J. Bartlett, M. Musiał, Rev. Mod. Phys. 2007, 79, 291.
- 34K. Raghavachari, G. W. Trucks, J. A. Pople, M. Head-Gordon, Chem. Phys. Lett. 1989, 157, 479–483.
- 35T. J. Lee, P. R. Taylor, Int. J. Quantum Chem. 1989, 36, 199–207.
- 36Gaussian 16, Revision B.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, et al., Gaussian, Inc. Wallingford CT, 2016.
- 37K. B. Wiberg, Tetrahedron 1968, 24, 1083–1096.
- 38A. E. Reed, R. B. Weinstock, F. Weinhold, J. Chem. Phys. 1985, 83, 735–746.
- 39E. D. Glendening, C. R. Landis, F. Weinhold, NBO 6.0. Theoretical Chemistry Institute, University of Wisconsin, Madison, WI, 2013.
- 40D. Y. Zubarev, A. I. Boldyrev, Phys. Chem. Chem. Phys. 2008, 10, 5207–5217.
- 41D. Y. Zubarev, A. I. Boldyrev, J. Org. Chem. 2008, 73, 9251–9258.
- 42T. A. Keith, TK Gristmill Software, Overland Parks, USA, 2019.
- 43P. Pyykkö, J. Phys. Chem. A 2015, 119, 2326–2337.
- 44S. Alvarez, Dalton Trans. 2013, 42, 8617–8636.
- 45C. Foroutan-Nejad, Angew. Chem. Int. Ed. 2020, 59, 20900–20903; Angew. Chem. 2020, 132, 21086–21089.
- 46R. Hoffmann, H. Hopf, Angew. Chem. Int. Ed. 2008, 47, 4474–4481; Angew. Chem. 2008, 120, 4548–4556.