Stabilization of Polynitrogen Anions in Tantalum–Nitrogen Compounds at High Pressure
Corresponding Author
Dr. Maxim Bykov
Department of Mathematics, Howard University, Washington, DC, 20059 USA
The Earth and Planets Laboratory, Carnegie Institution for Science, Washington, DC, 20015 USA
Search for more papers by this authorDr. Elena Bykova
The Earth and Planets Laboratory, Carnegie Institution for Science, Washington, DC, 20015 USA
Search for more papers by this authorDr. Alena V. Ponomareva
Materials Modeling and Development Laboratory, National University of Science and Technology 'MISIS', 119049 Moscow, Russia
Search for more papers by this authorProf. Dr. Igor A. Abrikosov
Department of Physics, Chemistry and Biology (IFM), Linköping University, 58183 Linköping, Sweden
Search for more papers by this authorDr. Stella Chariton
Center for Advanced Radiation Sources, University of Chicago, Lemont, IL, 60437 USA
Search for more papers by this authorDr. Vitali B. Prakapenka
Center for Advanced Radiation Sources, University of Chicago, Lemont, IL, 60437 USA
Search for more papers by this authorProf. Dr. Mohammad F. Mahmood
Department of Mathematics, Howard University, Washington, DC, 20059 USA
Search for more papers by this authorProf. Dr. Leonid Dubrovinsky
Bayerisches Geoinstitut, 95447 Bayreuth, Germany
Search for more papers by this authorDr. Alexander F. Goncharov
The Earth and Planets Laboratory, Carnegie Institution for Science, Washington, DC, 20015 USA
Search for more papers by this authorCorresponding Author
Dr. Maxim Bykov
Department of Mathematics, Howard University, Washington, DC, 20059 USA
The Earth and Planets Laboratory, Carnegie Institution for Science, Washington, DC, 20015 USA
Search for more papers by this authorDr. Elena Bykova
The Earth and Planets Laboratory, Carnegie Institution for Science, Washington, DC, 20015 USA
Search for more papers by this authorDr. Alena V. Ponomareva
Materials Modeling and Development Laboratory, National University of Science and Technology 'MISIS', 119049 Moscow, Russia
Search for more papers by this authorProf. Dr. Igor A. Abrikosov
Department of Physics, Chemistry and Biology (IFM), Linköping University, 58183 Linköping, Sweden
Search for more papers by this authorDr. Stella Chariton
Center for Advanced Radiation Sources, University of Chicago, Lemont, IL, 60437 USA
Search for more papers by this authorDr. Vitali B. Prakapenka
Center for Advanced Radiation Sources, University of Chicago, Lemont, IL, 60437 USA
Search for more papers by this authorProf. Dr. Mohammad F. Mahmood
Department of Mathematics, Howard University, Washington, DC, 20059 USA
Search for more papers by this authorProf. Dr. Leonid Dubrovinsky
Bayerisches Geoinstitut, 95447 Bayreuth, Germany
Search for more papers by this authorDr. Alexander F. Goncharov
The Earth and Planets Laboratory, Carnegie Institution for Science, Washington, DC, 20015 USA
Search for more papers by this authorGraphical Abstract
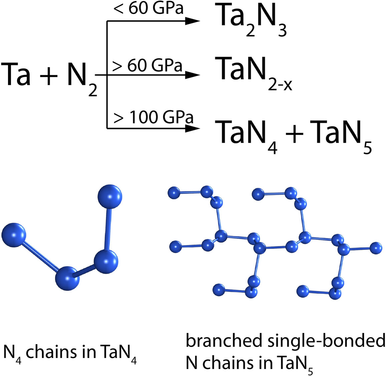
Direct reaction between nitrogen and tantalum at approximately 100 GPa results in the energetic nitrogen-rich compounds TaN4 and TaN5, which feature N4 chains and unprecedented branched single-bonded polymeric nitrogen chains in the crystal structures, respectively. TaN5 possesses an extraordinary volumetric energy density of 18 kJ cm−3, exceeding well-known high-energy-density materials.
Abstract
The synthesis of polynitrogen compounds is of great importance due to their potential as high-energy-density materials (HEDM), but because of the intrinsic instability of these compounds, their synthesis and stabilization is a fundamental challenge. Polymeric nitrogen units which may be stabilized in compounds with metals at high pressure are now restricted to non-branched chains with an average N−N bond order of 1.25, limiting their HEDM performances. Herein, we demonstrate the synthesis of a novel polynitrogen compound TaN5 via a direct reaction between tantalum and nitrogen in a diamond anvil cell at circa 100 GPa. TaN5 is the first example of a material containing branched all-single-bonded nitrogen chains [N55−]∞. Apart from that we discover two novel Ta–N compounds: TaN4 with finite N44− chains and the incommensurately modulated compound TaN2−x, which is recoverable at ambient conditions.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202100283-sup-0001-misc_information.pdf5.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. Curtius, Ber. Dtsch. Chem. Ges. 1890, 23, 3023–3033.
10.1002/cber.189002302232 Google Scholar
- 2G. Auffermann, Y. Prots, R. Kniep, Angew. Chem. Int. Ed. 2001, 40, 547–549;
10.1002/1521-3773(20010202)40:3<547::AID-ANIE547>3.0.CO;2-X CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 565–567.
- 3J. C. Crowhurst, A. F. Goncharov, B. Sadigh, J. M. Zaug, D. Aberg, Y. Meng, V. B. Prakapenka, J. Mater. Res. 2008, 23, 1–5.
- 4C. Zhang, C. Yang, B. Hu, C. Yu, Z. Zheng, C. Sun, Angew. Chem. Int. Ed. 2017, 56, 4512–4514; Angew. Chem. 2017, 129, 4583–4585.
- 5Y. Xu, Q. Wang, C. Shen, Q. Lin, P. Wang, M. Lu, Nature 2017, 549, 78–81.
- 6K. O. Christe, W. W. Wilson, J. A. Sheehy, J. A. Boatz, Angew. Chem. Int. Ed. 1999, 38, 2004–2009;
10.1002/(SICI)1521-3773(19990712)38:13/14<2004::AID-ANIE2004>3.0.CO;2-7 CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 2112–2118.10.1002/(SICI)1521-3757(19990712)111:13/14<2112::AID-ANGE2112>3.0.CO;2-I Web of Science® Google Scholar
- 7D. Laniel, B. Winkler, E. Koemets, T. Fedotenko, M. Bykov, E. Bykova, L. Dubrovinsky, N. Dubrovinskaia, Nat. Commun. 2019, 10, 4515.
- 8M. I. Eremets, A. G. Gavriliuk, I. A. Trojan, D. A. Dzivenko, R. Boehler, Nat. Mater. 2004, 3, 558–563.
- 9D. Laniel, B. Winkler, T. Fedotenko, A. Pakhomova, S. Chariton, V. Milman, V. Prakapenka, L. Dubrovinsky, N. Dubrovinskaia, Phys. Rev. Lett. 2020, 124, 216001.
- 10B. A. Steele, E. Stavrou, J. C. Crowhurst, J. M. Zaug, V. B. Prakapenka, I. I. Oleynik, Chem. Mater. 2017, 29, 735–741.
- 11D. Laniel, G. Weck, G. Gaiffe, G. Garbarino, P. Loubeyre, J. Phys. Chem. Lett. 2018, 9, 1600–1604.
- 12M. Bykov, T. Fedotenko, S. Chariton, D. Laniel, K. Glazyrin, M. Hanfland, J. S. Smith, V. B. Prakapenka, M. F. Mahmood, A. F. Goncharov, et al., arXiv:2010.15774.
- 13M. Bykov, E. Bykova, G. Aprilis, K. Glazyrin, E. Koemets, I. Chuvashova, I. Kupenko, C. McCammon, M. Mezouar, V. Prakapenka, et al., Nat. Commun. 2018, 9, 2756.
- 14M. Bykov, S. Khandarkhaeva, T. Fedotenko, P. Sedmak, N. Dubrovinskaia, L. Dubrovinsky, Acta Crystallogr. Sect. E 2018, 74, 1392–1395.
- 15J. Zhang, A. R. Oganov, X. Li, H. Niu, Phys. Rev. B 2017, 95, 020103.
- 16A. G. Kvashnin, A. R. Oganov, A. I. Samtsevich, Z. Allahyari, J. Phys. Chem. Lett. 2017, 8, 755–764.
- 17B. A. Steele, I. I. Oleynik, Chem. Phys. Lett. 2016, 643, 21–26.
- 18S. Wei, D. Li, Z. Liu, X. Li, F. Tian, D. Duan, B. Liu, T. Cui, Phys. Chem. Chem. Phys. 2017, 19, 9246–9252.
- 19Z. Zhao, K. Bao, D. Li, D. Duan, F. Tian, X. Jin, C. Chen, X. Huang, B. Liu, T. Cui, Sci. Rep. 2014, 4, 4797.
- 20M. Bykov, E. Bykova, E. Koemets, T. Fedotenko, G. Aprilis, K. Glazyrin, H.-P. P. Liermann, A. V. Ponomareva, J. Tidholm, F. Tasnádi, et al., Angew. Chem. Int. Ed. 2018, 57, 9048–9053; Angew. Chem. 2018, 130, 9186–9191.
- 21M. Bykov, S. Chariton, E. Bykova, S. Khandarkhaeva, T. Fedotenko, A. V. Ponomareva, J. Tidholm, F. Tasnádi, I. A. Abrikosov, P. Sedmak, et al., Angew. Chem. Int. Ed. 2020, 59, 10321–10326; Angew. Chem. 2020, 132, 10407–10412.
- 22P. Höhn, R. Niewa, Handbook of Solid State Chemistry, Wiley-VCH, Weinheim, 2017, pp. 251–359.
10.1002/9783527691036.hsscvol1008 Google Scholar
- 23N. Schönberg, W. G. Overend, A. Munthe-Kaas, N. A. Sörensen, Acta Chem. Scand. 1954, 8, 199–203.
- 24L. E. Conroy, A. N. Christensen, J. Solid State Chem. 1977, 20, 205–207.
- 25A. Fontbonne, J.-C. Gilles, Rev. Int. Hautes Temp. Refract. 1969, 6, 181–191.
- 26A. Zerr, G. Miehe, J. Li, D. A. Dzivenko, V. K. Bulatov, H. Höfer, N. Boifan-Casanova, M. Fialin, G. Brey, T. Watanabe, et al., Adv. Funct. Mater. 2009, 19, 2282–2288.
- 27A. Y. Ganin, L. Kienle, G. V. Vajenine, Eur. J. Inorg. Chem. 2004, 3233–3239.
- 28J. Strähle, Z. Anorg. Allg. Chem. 1973, 402, 47–57.
- 29A. Salamat, K. Woodhead, S. I. U. Shah, A. L. Hector, P. F. McMillan, Chem. Commun. 2014, 50, 10041–10044.
- 30P. Kroll, T. Schröter, M. Peters, Angew. Chem. Int. Ed. 2005, 44, 4249–4254; Angew. Chem. 2005, 117, 4321–4326.
- 31A. Friedrich, B. Winkler, L. Bayarjargal, E. A. Juarez Arellano, W. Morgenroth, J. Biehler, F. Schröder, J. Yan, S. M. Clark, J. Alloys Compd. 2010, 502, 5–12.
- 32D. Li, F. Tian, D. Duan, K. Bao, B. Chu, X. Sha, B. Liu, T. Cui, RSC Adv. 2014, 4, 10133–10139.
- 33H. Yan, M. Zhang, Q. Wei, P. Guo, J. Alloys Compd. 2013, 581, 508–514.
- 34S. K. R. Patil, N. S. Mangale, S. V. Khare, S. Marsillac, Thin Solid Films 2008, 517, 824–827.
- 35G. Soto, Comput. Mater. Sci. 2012, 61, 1–5.
- 36H. Alkhaldi, P. Kroll, J. Phys. Chem. C 2020, 124, 22221–22227.
- 37M. Bykov, S. Chariton, H. Fei, T. Fedotenko, G. Aprilis, A. V. Ponomareva, F. Tasnádi, I. A. Abrikosov, B. Merle, P. Feldner, et al., Nat. Commun. 2019, 10, 2994.
- 38Deposition Numbers 2038294, 2038295, and 2038296 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 39R. Nesper, Z. Anorg. Allg. Chem. 2014, 640, 2639–2648.
- 40W. J. Schutte, J. L. de Boer, Acta Crystallogr. Sect. B 1993, 49, 398–403.
- 41M. Bykov, E. Bykova, L. Dubrovinsky, M. Hanfland, H.-P. Liermann, S. van Smaalen, Sci. Rep. 2015, 5, 9647.
- 42M. Bykov, J. Zhang, A. Schönleber, A. Wölfel, Phys. Rev. B 2013, 88, 184420.
- 43M. Bykov, E. Bykova, M. Hanfland, H.-P. Liermann, R. K. Kremer, R. Glaum, L. Dubrovinsky, S. van Smaalen, Angew. Chem. Int. Ed. 2016, 55, 15053–15057; Angew. Chem. 2016, 128, 15277–15281.
- 44V. L. Moruzzi, J. F. Janak, K. Schwarz, Phys. Rev. B 1988, 37, 790–799.
- 45 JANAF Thermo- Chemical Tables (Eds.: D. R. Stull, H. Prophet), National Bureau Of Standards, Gaithersburg, 1971.
- 46B. M. Dobratz, LLNL Explosives Handbook: Properties of Chemical Explosives and Explosives and Explosive Simulants, 1981.
- 47A. F. Young, C. Sanloup, E. Gregoryanz, S. Scandolo, R. J. Hemley, H. Mao, Phys. Rev. Lett. 2006, 96, 155501.
- 48M. Bykov, K. V. Yusenko, E. Bykova, A. Pakhomova, W. Kraus, N. Dubrovinskaia, L. Dubrovinsky, Eur. J. Inorg. Chem. 2019, 3667–3671.
- 49K. Niwa, H. Ogasawara, M. Hasegawa, Dalton Trans. 2017, 46, 9750–9754.
- 50G. V. Vajenine, G. Auffermann, Y. Prots, W. Schnelle, R. K. Kremer, A. Simon, R. Kniep, Inorg. Chem. 2001, 40, 4866–4870.
- 51M. Bykov, K. R. Tasca, I. G. Batyrev, D. Smith, K. Glazyrin, S. Chariton, M. Mahmood, A. F. Goncharov, Inorg. Chem. 2020, 59, 14819–14826.