Pd/Cu-Catalyzed Defluorinative Carbonylative Coupling of Aryl Iodides and gem-Difluoroalkenes: Efficient Synthesis of α-Fluorochalcones
Fu-Peng Wu
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorYang Yuan
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Jiawang Liu
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao-Feng Wu
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, Liaoning, China
Search for more papers by this authorFu-Peng Wu
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorYang Yuan
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Jiawang Liu
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao-Feng Wu
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, Liaoning, China
Search for more papers by this authorDedicated to Professor Ilhyong Ryu on the occasion of his 70th birthday
Graphical Abstract
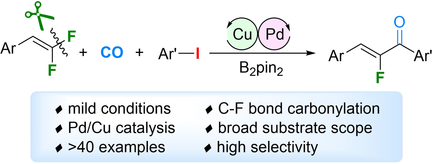
Enabled by a Pd/Cu cooperative catalyst system, the first example of defluorinative carbonylative coupling has been established. With gem-difluoroalkenes and aryl iodides as the substrates, this methodology offers flexible and facile access to privileged α-fluorochalcones under mild reaction conditions in moderate-to-excellent yields.
Abstract
An unprecedented and challenging defluorinative carbonylation was achieved. Enabled by a Pd/Cu cooperative catalyst system, the first example of defluorinative carbonylative coupling has been established. With gem-difluoroalkenes and aryl iodides as the substrates, this methodology offers flexible and facile access to privileged α-fluorochalcones under mild reaction conditions in moderate-to-excellent yields. Mechanistic studies indicated transmetalation between palladium and copper intermediates as a crucial step of the catalytic cycle.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202017365-sup-0001-misc_information.pdf7.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. K. Hagmann, J. Med. Chem. 2008, 51, 4359–4369;
- 1bK. Muller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 1cT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. Int. Ed. 2013, 52, 8214–8264; Angew. Chem. 2013, 125, 8372–8423.
- 2
- 2aY. Asahina, K. Iwase, F. Iinuma, M. Hosaka, T. Ishizaki, J. Med. Chem. 2005, 48, 3194–3202;
- 2bS. Couve-Bonnaire, D. Cahard, X. Pannecoucke, Org. Biomol. Chem. 2007, 5, 1151–1157;
- 2cC. E. Jakobsche, A. Choudhary, S. J. Miller, R. T. Raines, J. Am. Chem. Soc. 2010, 132, 6651–6653;
- 2dS. Osada, S. Sano, M. Ueyama, Y. Chuman, H. Kodama, K. Sakaguchi, Bioorg. Med. Chem. 2010, 18, 605–611.
- 3
- 3aG. Landelle, M. Bergeron, M. O. Turcotte-Savard, J. F. Paquin, Chem. Soc. Rev. 2011, 40, 2867–2908;
- 3bH. Yanai, T. Taguchi, Eur. J. Org. Chem. 2011, 5939–5954.
- 4Recent reviews on synthesis of gem-difluoroalkenes:
- 4aX. Zhang, S. Cao, Tetrahedron Lett. 2017, 58, 375–392;
- 4bC. Liu, H. Zeng, C. Zhu, H. Jiang, Chem. Commun. 2020, 56, 10442–10452;
- 4cS. Koley, R. A. Altman, Isr. J. Chem. 2020, 60, 313–339.
- 5X. Lu, Y. Wang, B. Zhang, J. J. Pi, X. X. Wang, T. J. Gong, B. Xiao, Y. Fu, J. Am. Chem. Soc. 2017, 139, 12632–12637.
- 6Recent examples, see:
- 6aM. Ohashi, R. Kamura, R. Doi, S. Ogoshi, Chem. Lett. 2013, 42, 933–935;
- 6bW. Dai, H. Shi, X. Zhao, S. Cao, Org. Lett. 2016, 18, 4284–4287;
- 6cJ. Zhang, C. Xu, W. Wu, S. Cao, Chem. Eur. J. 2016, 22, 9902–9908;
- 6dZ. S. Cong, Y. G. Li, L. Chen, F. Xing, G. F. Du, C. Z. Gu, L. He, Org. Biomol. Chem. 2017, 15, 3863–3868.
- 7Recent examples, see:
- 7aL. Kong, X. Zhou, X. Li, Org. Lett. 2016, 18, 6320–6323;
- 7bD. Zell, U. Dhawa, V. Müller, M. Bursch, S. Grimme, L. Ackermann, ACS Catal. 2017, 7, 4209–4213;
- 7cS. H. Cai, L. Ye, D. X. Wang, Y. Q. Wang, L. J. Lai, C. Zhu, C. Feng, T. P. Loh, Chem. Commun. 2017, 53, 8731–8734;
- 7dP. Tian, C. Feng, T.-P. Loh, Nat. Commun. 2015, 6, 7472;
- 7eJ. Hu, X. Han, Y. Yuan, Z. Shi, Angew. Chem. Int. Ed. 2017, 56, 13342–13346; Angew. Chem. 2017, 129, 13527–13531;
- 7fR. T. Thornbury, F. D. Toste, Angew. Chem. Int. Ed. 2016, 55, 11629–11632; Angew. Chem. 2016, 128, 11801–11804;
- 7gJ. Xie, J. Yu, M. Rudolph, F. Rominger, A. S. Hashmi, Angew. Chem. Int. Ed. 2016, 55, 9416–9421; Angew. Chem. 2016, 128, 9563–9568;
- 7hY. Xiong, T. Huang, X. Ji, J. Wu, S. Cao, Org. Biomol. Chem. 2015, 13, 7389–7392;
- 7iR. Kojima, K. Kubota, H. Ito, Chem. Commun. 2017, 53, 10688–10691;
- 7jN. O. Andrella, N. Xu, B. M. Gabidullin, C. Ehm, R. T. Baker, J. Am. Chem. Soc. 2019, 141, 11506–11521;
- 7kH. Sakaguchi, Y. Uetake, M. Ohashi, T. Niwa, S. Ogoshi, T. Hosoya, J. Am. Chem. Soc. 2017, 139, 12855–12862;
- 7lH. W. Du, J. Sun, Q. S. Gao, J. Y. Wang, H. Wang, Z. Xu, M. D. Zhou, Org. Lett. 2020, 22, 1542–1546;
- 7mA. Kondoh, K. Koda, M. Terada, Org. Lett. 2019, 21, 2277–2280;
- 7nJ. Zhang, W. Dai, Q. Liu, S. Cao, Org. Lett. 2017, 19, 3283–3286;
- 7oQ. Ma, C. Liu, G. C. Tsui, Org. Lett. 2020, 22, 5193–5197;
- 7pQ. Ma, Y. Wang, G. C. Tsui, Angew. Chem. Int. Ed. 2020, 59, 11293–11297; Angew. Chem. 2020, 132, 11389–11393;
- 7qT. Fujita, K. Fuchibe, J. Ichikawa, Angew. Chem. Int. Ed. 2019, 58, 390–402; Angew. Chem. 2019, 131, 396–408.
- 8Recent example:
- 8aZ. Lin, Y. Lan, C. Wang, ACS Catal. 2019, 9, 775–780;
- 8bT. Ma, Y. Chen, Y. Li, Y. Ping, W. Kong, ACS Catal. 2019, 9, 9127–9133;
- 8cJ. Li, W. Rao, S.-Y. Wang, S.-J. Ji, J. Org. Chem. 2019, 84, 11542–11552.
- 9Recent example:
- 9aS.-S. Yan, D.-S. Wu, J.-H. Ye, L. Gong, X. Zeng, C.-K. Ran, Y.-Y. Gui, J. Li, D.-G. Yu, ACS Catal. 2019, 9, 6987–6992;
- 9bJ. Liu, W. Nie, H. Yu, J. Shi, Org. Biomol. Chem. 2020, 18, 9065–9071.
- 10
- 10aX.-F. Wu, H. Neumann, M. Beller, Chem. Rev. 2013, 113, 1–35;
- 10bJ.-B. Peng, F.-P. Wu, X.-F. Wu, Chem. Rev. 2019, 119, 2090–2127;
- 10cY. Li, Y. Hu, X.-F. Wu, Chem. Soc. Rev. 2018, 47, 172–194.
- 11Selective reviews and examples of C−F bond activation:
- 11aH. Amii, K. Uneyama, Chem. Rev. 2009, 109, 2119–2183;
- 11bT. Ahrens, J. Kohlmann, M. Ahrens, T. Braun, Chem. Rev. 2015, 115, 931–972;
- 11cM. Ohashi, H. Saijo, M. Shibata, S. Ogoshi, Eur. J. Org. Chem. 2013, 443–447.
- 12J. Vela, J. Smith, Y. Yu, N. Ketterer, C. Flaschenriem, R. Lachicotte, P. Holland, J. Am. Chem. Soc. 2005, 127, 7857–7870.
- 13
- 13aX.-F. Wu, H. Neumann, A. Spannenberg, T. Schulz, H. Jiao, M. Beller, J. Am. Chem. Soc. 2010, 132, 14596–14602;
- 13bX.-F. Wu, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 2010, 49, 5284–5288; Angew. Chem. 2010, 122, 5412–5416;
- 13cJ. Schranck, X.-F. Wu, H. Neumann, M. Beller, Chem. Eur. J. 2012, 18, 4827–4831;
- 13dK. S. Bloome, E. J. Alexanian, J. Am. Chem. Soc. 2010, 132, 12823–12825;
- 13eM. K. Brennaman, A. O. T. Patrocinio, W. Song, J. W. Jurss, J. J. Concepcion, P. G. Hoertz, M. C. Traub, N. Y. Murakami Iha, T. J. Meyer, ChemSusChem 2011, 4, 216–227;
- 13fX.-F. Wu, H. Neumann, M. Beller, Chem. Asian J. 2012, 7, 282–285.
- 14
- 14a“Peptidomimetics”: G. Klebe in Drug Design (Eds.: G. Klebe), Springer, Berlin, 2013;
10.1007/978-3-642-17907-5 Google Scholar
- 14bR. S. H. Liu, H. Matsumoto, A. E. Asato, M. Denny, Y. Shichida, T. Yoshizawa, F. W. Dahlquist, J. Am. Chem. Soc. 1981, 103, 7195–7201.
- 15
- 15aJ. Qian, W. Yi, X. Huang, Y. Miao, J. Zhang, C. Cai, W. Zhang, Org. Lett. 2015, 17, 1090–1093;
- 15bY. Bessière, D. N.-H. Savary, M. Schlosser, Helv. Chim. Acta 1977, 60, 1739–1746;
- 15cT. Allmendinger, P. Furet, E. Hungerbühler, Tetrahedron Lett. 1990, 31, 7297–7300;
- 15dT. Allmendinger, E. Felder, E. Hungarbühler, Tetrahedron Lett. 1990, 31, 7301–7304.