Formation of Nanoscale [Ge4O16Al48(OH)108(H2O)24]20+ from Condensation of ϵ-GeAl128+ Keggin Polycations**
Mohammad Shohel
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorJennifer L. Bjorklund
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorJack A. Smith
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorDmytro V. Kravchuk
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorDr. Sara E. Mason
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorCorresponding Author
Dr. Tori Z. Forbes
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorMohammad Shohel
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorJennifer L. Bjorklund
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorJack A. Smith
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorDmytro V. Kravchuk
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorDr. Sara E. Mason
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorCorresponding Author
Dr. Tori Z. Forbes
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorA previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv.12910130.v1).
Graphical Abstract
Abstract
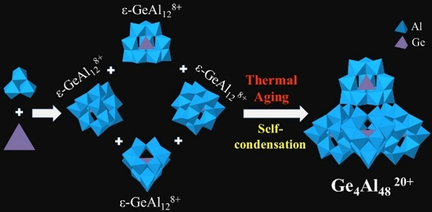
Keggin-type polyaluminum cations belong to a unique class of compounds with their large positive charge, hydroxo bridges, and divergent isomerization/oligomerization. Previous reports indicated that oligomerization of this species can only occur through one isomer (δ), but herein we report the isolation of largest Keggin-type cluster that occurs through self-condensation of four ϵ-isomers ϵ-GeAl128+ to form [Ge4O16Al48(OH)108(H2O)24]20+ cluster (Ge4Al48). The cluster was crystallized and structurally characterized by single-crystal X-ray diffraction (SCXRD) and the elemental composition was confirmed by ICP-MS and SEM-EDS. Additional dynamic light scattering experiments confirms the presence of the Ge4Al48 in thermally aged solutions. DFT calculations reveal that a single atom Ge substitution in tetrahedral site of ϵ-isomer is the key for the formation of Ge4Al48 because it activates deprotonation at key surface sites that control the self-condensation process.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202017321-sup-0001-misc_information.pdf1.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. F. Keggin, Nature 1933, 131, 908–909.
- 2D. Li, P. Ma, J. Niu, J. Wang, Coord. Chem. Rev. 2019, 392, 49–80.
- 3
- 3aW. O. N. Parker, R. Millini, I. Kiricsi, Inorg. Chem. 1997, 36, 571–575;
- 3bA. P. Lee, B. L. Phillips, M. M. Olmstead, W. H. Casey, Inorg. Chem. 2001, 40, 4485–4487;
- 3cM. Shohel, J. L. Bjorklund, E. A. Ovrom, S. E. Mason, T. Z. Forbes, Inorg. Chem. 2020, 59, 10461–10472;
- 3dW. Wang, L. B. Fullmer, N. A. G. Bandeira, S. Goberna-Ferrón, L. N. Zakharov, C. Bo, D. A. Keszler, M. Nyman, Chem 2016, 1, 887–901;
- 3eW. Wang, M. Amiri, K. Kozma, J. Lu, L. N. Zakharov, M. Nyman, Eur. J. Inorg. Chem. 2018, 4638–4642.
- 4
- 4aM. Muñoz, G. Romanelli, I. L. Botto, C. I. Cabello, C. Lamonier, M. Capron, P. Baranek, P. Blanchard, E. Payen, Appl. Catal. B 2010, 100, 254–263;
- 4bI. Benito, A. del Riego, M. Martínez, C. Blanco, C. Pesquera, F. González, Appl. Catal. A 1999, 180, 175–182;
- 4cT. A. Stewart, D. E. Trudell, T. M. Alam, C. A. Ohlin, C. Lawler, W. H. Casey, S. Jett, M. Nyman, Environ. Sci. Technol. 2009, 43, 5416–5422.
- 5A. Bijelic, M. Aureliano, A. Rompel, Angew. Chem. Int. Ed. 2019, 58, 2980–2999; Angew. Chem. 2019, 131, 3008–3029.
- 6
- 6aH. J. Yeo, Y. Paik, S.-M. Paek, I. Honma, J. Phys. Chem. Solids 2012, 73, 1417–1419;
- 6bM. Priyadarshini, S. Shanmugan, K. P. Kirubakaran, A. Thomas, M. Prakash, C. Senthil, C. W. Lee, K. Vediappan, J. Phys. Chem. Solids 2020, 142, 109468.
- 7G. Wang, J. Zhou, J. Li, Biosens. Bioelectron. 2007, 22, 2921–2925.
- 8X. Chen, P. Huang, X. Zhu, S. Zhuang, H. Zhu, J. Fu, A. S. Nissimagoudar, W. Li, X. Zhang, L. Zhou, Y. Wang, Z. Lv, Y. Zhou, S.-T. Han, Nanoscale Horiz. 2019, 4, 697–704.
- 9
- 9aG. Furrer, B. L. Phillips, K.-U. Ulrich, R. Pöthig, W. H. Casey, Science 2002, 297, 2245;
- 9bO. Sadeghi, L. N. Zakharov, M. Nyman, Science 2015, 347, 1359;
- 9cM. Nyman in Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth (Ed.: W. M. White), Springer International Publishing, Cham, 2016, pp. 1–5.
- 10D.-L. Long, E. Burkholder, L. Cronin, Chem. Soc. Rev. 2007, 36, 105–121.
- 11
- 11aS. Abeysinghe, D. K. Unruh, T. Z. Forbes, Cryst. Growth Des. 2012, 12, 2044–2051;
- 11bZ. Sun, H. Wang, H. Tong, S. Sun, Inorg. Chem. 2011, 50, 559–564.
- 12P. Mothé-Esteves, M. M. Pereira, J. Arichi, B. Louis, Cryst. Growth Des. 2010, 10, 371–378.
- 13
- 13aS. Matsunaga, Y. Inoue, K. Mihara, K. Nomiya, Inorg. Chem. Commun. 2017, 80, 61–64;
- 13bG. A. Al-Kadamany, F. Hussain, S. S. Mal, M. H. Dickman, N. Leclerc-Laronze, J. Marrot, E. Cadot, U. Kortz, Inorg. Chem. 2008, 47, 8574–8576.
- 14
- 14aY. Sakai, K. Yoza, C. N. Kato, K. Nomiya, Chem. Eur. J. 2003, 9, 4077–4083;
- 14bY. Sakai, S. Ohta, Y. Shintoyo, S. Yoshida, Y. Taguchi, Y. Matsuki, S. Matsunaga, K. Nomiya, Inorg. Chem. 2011, 50, 6575–6583;
- 14cZ. Zhang, Y.-L. Wang, G.-Y. Yang, Acta Crystallogr. Sect. C 2018, 74, 1284–1288;
- 14dJ.-W. Zhao, J. Zhang, S.-T. Zheng, G.-Y. Yang, Inorg. Chem. 2007, 46, 10944–10946;
- 14eK.-Y. Wang, B. S. Bassil, Z.-G. Lin, A. Haider, J. Cao, H. Stephan, K. Viehweger, U. Kortz, Dalton Trans. 2014, 43, 16143–16146;
- 14fC. P. Pradeep, D.-L. Long, P. Kögerler, L. Cronin, Chem. Commun. 2007, 4254–4256;
- 14gS. Yoshitaka, Y. Shoko, H. Takeshi, M. Hideyuki, N. Kenji, Bull. Chem. Soc. Jpn. 2007, 80, 1965–1974.
- 15J. L. Bjorklund, J. W. Bennett, T. Z. Forbes, S. E. Mason, Cryst. Growth Des. 2019, 19, 2820–2829.
- 16A. P. Lee, G. Furrer, W. H. Casey, J. Colloid Interface Sci. 2002, 250, 269–270.
- 17Y. Sakai, K. Yoza, C. N. Kato, K. Nomiya, Dalton Trans. 2003, 3581–3586.
- 18
- 18aG. Al-Kadamany, Jacobs University (IRC-Library, Information Resource Center der Jacobs University Bremen), 2010;
- 18bG.-S. Kim, H. Zeng, D. VanDerveer, C. L. Hill, Angew. Chem. Int. Ed. 1999, 38, 3205–3207;
10.1002/(SICI)1521-3773(19991102)38:21<3205::AID-ANIE3205>3.0.CO;2-U CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 3413–3416.10.1002/(SICI)1521-3757(19991102)111:21<3413::AID-ANGE3413>3.0.CO;2-O Web of Science® Google Scholar
- 19F. Hussain, B. S. Bassil, L.-H. Bi, M. Reicke, U. Kortz, Angew. Chem. Int. Ed. 2004, 43, 3485–3488; Angew. Chem. 2004, 116, 3567–3571.
- 20J. W. Bennett, J. L. Bjorklund, T. Z. Forbes, S. E. Mason, Inorg. Chem. 2017, 56, 13014–13028.
- 21T. C. J. Towns, M. Dahan, I. Foster, K. Gaither, A. Grimshaw, V. Hazlewood, S. Lathrop, D. Lifka, G. D. Peterson, R. Roskies, J. R. Scott, N. Wilkins-Diehr, Comput. Sci. Eng. 2014, 16, 62–74.