Metal-Organic Cages with Missing Linker Defects
Xianhui Tang
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorDandan Chu
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorWei Gong
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorProf. Yong Cui
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Prof. Yan Liu
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorXianhui Tang
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorDandan Chu
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorWei Gong
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorProf. Yong Cui
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Prof. Yan Liu
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules and State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorGraphical Abstract
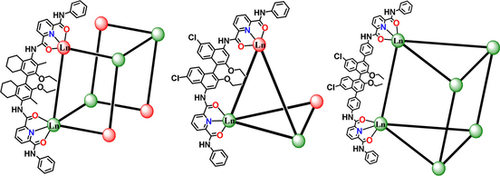
Tetrahedral and hexahedral coordination cages with one or two missing linkers, as well as regular trigonal prismatic cages, are synthesized by employing steric hindrance of organic linkers to manipulate coordination modes of lanthanide(III) ions. The defective cages, especially the hexahedral cage, are conformationally flexible and adapt its structure to accommodate various guest molecules with sizes comparable or much larger than the cavity portals that are not accessible to the non-defective cage.
Abstract
We present here the controlled synthesis of defective coordination cages by employing steric hindrance of organic linkers to manipulate coordination modes of the assembled metal ions. Three chiral 1,1′-bi-2-naphthol (BINOL) derived bis-tridentate ligands L1–L3 with pyridine-2,6-dicarboxamides (pcam) chelating moieties are therefore designed and synthesized, among which L3 has a smaller steric hindrance on the coordinating sites relative to the other two linkers. Complexes of L1 and L2 with lanthanides afford the irregular Ln8(L1)10 hexahedra with two missing edges and Ln4(L2)5 tetrahedra with one missing edge, respectively, both of which contain a 1:1 mixture of Ln(pcam)2 and Ln(pcam)3. In contrast, complex of L3 produces the regular twisted Ln6(L3)9 trigonal prisms without missing edges that contain only Ln(pcam)3 vertices. The defective cage has more freedom to adjust its structural conformation, affording adaptable cavity to accommodate a range of guest molecules with sizes comparable or much larger than the cavity portals.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202017244-sup-0001-misc_information.pdf8.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. J. Brown, R. G. Bergman, K. N. Raymond, J. Am. Chem. Soc. 2009, 131, 17530–17531;
- 1bY. Qiao, L. Zhang, J. Li, W. Lin, Z. Wang, Angew. Chem. Int. Ed. 2016, 55, 12778–12782; Angew. Chem. 2016, 128, 12970–12974;
- 1cW. Cullen, M. C. Misuraca, C. A. Hunter, N. H. Williams, M. D. Ward, Nat. Chem. 2016, 8, 231–236;
- 1dB. P. Burke, W. Grantham, M. J. Burke, G. S. Nichol, D. Roberts, I. Renard, R. Hargreaves, C. Cawthorne, S. J. Archibald, P. J. Lusby, J. Am. Chem. Soc. 2018, 140, 16877–16881;
- 1eJ. E. M. Lewis, E. L. Gavey, S. A. Cameron, J. D. Crowley, Chem. Sci. 2012, 3, 778–784;
- 1fL. R. Holloway, P. M. Bogie, Y. Lyon, C. Ngai, T. F. Miller, R. R. Julian, R. J. Hooley, J. Am. Chem. Soc. 2018, 140, 8078–8081.
- 2
- 2aT. R. Cook, P. J. Stang, Chem. Rev. 2015, 115, 7001–7045;
- 2bH. Wang, C. H. Liu, K. Wang, M. Wang, H. Yu, S. Kandapal, R. Brzozowski, B. Xu, M. Wang, S. Lu, X. Q. Hao, P. Eswara, M. P. Nieh, J. Cai, X. Li, J. Am. Chem. Soc. 2019, 141, 16108–16116;
- 2cX. P. Zhou, J. Liu, S. Z. Zhan, J. R. Yang, D. Li, K. M. Ng, R. W. Sun, C. M. Che, J. Am. Chem. Soc. 2012, 134, 8042–8045;
- 2dH. Li, Y. F. Han, Y. J. Lin, Z. W. Guo, G. X. Jin, J. Am. Chem. Soc. 2014, 136, 2982–2985;
- 2eG. Liu, Y. D. Yuan, J. Wang, Y. Cheng, S. B. Peh, Y. Wang, Y. Qian, J. Dong, D. Yuan, D. Zhao, J. Am. Chem. Soc. 2018, 140, 6231–6234.
- 3
- 3aM. Yoshizawa, J. Nakagawa, K. Kumazawa, M. Nagao, M. Kawano, T. Ozeki, M. Fujita, Angew. Chem. Int. Ed. 2005, 44, 1810–1813; Angew. Chem. 2005, 117, 1844–1847;
- 3bY. Shi, K. Cai, H. Xiao, Z. Liu, J. Zhou, D. Shen, Y. Qiu, Q. H. Guo, C. Stern, M. R. Wasielewski, F. Diederich, W. A. Goddard 3rd, J. F. Stoddart, J. Am. Chem. Soc. 2018, 140, 13835–13842;
- 3cR. Saha, A. Devaraj, S. Bhattacharyya, S. Das, E. Zangrando, P. S. Mukherjee, J. Am. Chem. Soc. 2019, 141, 8638–8645;
- 3dX. Yan, M. Wang, T. R. Cook, M. Zhang, M. L. Saha, Z. Zhou, X. Li, F. Huang, P. J. Stang, J. Am. Chem. Soc. 2016, 138, 4580–4588;
- 3eJ. Jiao, Z. Li, Z. Qiao, X. Li, Y. Liu, J. Dong, J. Jiang, Y. Cui, Nat. Commun. 2018, 9, 4423;
- 3fJ. Dong, C. Tan, K. Zhang, Y. Liu, P. J. Low, J. Jiang, Y. Cui, J. Am. Chem. Soc. 2017, 139, 1554–1564.
- 4
- 4aE. J. Gosselin, G. E. Decker, A. M. Antonio, G. R. Lorzing, G. P. A. Yap, E. D. Bloch, J. Am. Chem. Soc. 2020, 142, 9594–9598;
- 4bD. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka, M. Fujita, Nature 2016, 540, 563–566;
- 4cL. Pesce, C. Perego, A. B. Grommet, R. Klajn, G. M. Pavan, J. Am. Chem. Soc. 2020, 142, 9792–9802;
- 4dD. M. Kaphan, M. D. Levin, R. G. Bergman, K. N. Raymond, F. D. Toste, Science 2015, 350, 1235–1238;
- 4eM. Yamashina, M. M. Sartin, Y. Sei, M. Akita, S. Takeuchi, T. Tahara, M. Yoshizawa, J. Am. Chem. Soc. 2015, 137, 9266–9269;
- 4fX. Jing, C. He, Y. Yang, C. Duan, J. Am. Chem. Soc. 2015, 137, 3967–3974.
- 5
- 5aK. Li, L. Y. Zhang, C. Yan, S. C. Wei, M. Pan, L. Zhang, C. Y. Su, J. Am. Chem. Soc. 2014, 136, 4456–4459;
- 5bW. Gong, D. Chu, H. Jiang, X. Chen, Y. Cui, Y. Liu, Nat. Commun. 2019, 10, 600;
- 5cC. Tan, J. Jiao, Z. Li, Y. Liu, X. Han, Y. Cui, Angew. Chem. Int. Ed. 2018, 57, 2085–2090; Angew. Chem. 2018, 130, 2107–2112;
- 5dJ. Jiao, C. Tan, Z. Li, Y. Liu, X. Han, Y. Cui, J. Am. Chem. Soc. 2018, 140, 2251–2259;
- 5eY. Ye, T. R. Cook, S. P. Wang, J. Wu, S. Li, P. J. Stang, J. Am. Chem. Soc. 2015, 137, 11896–11899;
- 5fF. J. Rizzuto, P. Prohm, A. J. Plajer, J. L. Greenfield, J. R. Nitschke, J. Am. Chem. Soc. 2019, 141, 1707–1715;
- 5gC. Gütz, R. Hovorka, C. Klein, Q. Q. Jiang, C. Bannwarth, M. Engeser, C. Schmuck, W. Assenmacher, W. Mader, F. Topić, K. Rissanen, S. Grimme, A. Lützen, Angew. Chem. Int. Ed. 2014, 53, 1693–1698; Angew. Chem. 2014, 126, 1719–1724.
- 6
- 6aM. L. Kuhlman, T. B. Rauchfuss, J. Am. Chem. Soc. 2003, 125, 10084–10092;
- 6bR. Zhu, W. M. Bloch, J. J. Holstein, S. Mandal, L. V. Schafer, G. H. Clever, Chem. Eur. J. 2018, 24, 12976–12982;
- 6cB. Chen, J. J. Holstein, S. Horiuchi, W. G. Hiller, G. H. Clever, J. Am. Chem. Soc. 2019, 141, 8907–8913.
- 7
- 7aZ. Fang, B. Bueken, D. E. De Vos, R. A. Fischer, Angew. Chem. Int. Ed. 2015, 54, 7234–7254; Angew. Chem. 2015, 127, 7340–7362;
- 7bH. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim, W. Zhou, J. Am. Chem. Soc. 2013, 135, 10525–10532.
- 8
- 8aJ. L. Lunkley, D. Shirotani, K. Yamanari, S. Kaizaki, G. Muller, J. Am. Chem. Soc. 2008, 130, 13814–13815;
- 8bY. Zhou, H. Li, T. Zhu, T. Gao, P. Yan, J. Am. Chem. Soc. 2019, 141, 19634–19643;
- 8cY. B. Tan, Y. Okayasu, S. Katao, Y. Nishikawa, F. Asanoma, M. Yamada, J. Yuasa, T. Kawai, J. Am. Chem. Soc. 2020, 142, 17653–17661.
- 9
- 9aB. E. Aroussi, S. Zebret, C. Besnard, P. Perrottet, J. Hamacek, J. Am. Chem. Soc. 2011, 133, 10764–10767;
- 9bL. Zhao, S. Qu, C. He, R. Zhang, C. Duan, Chem. Commun. 2011, 47, 9387–9389;
- 9cJ. Hamacek, G. Bernardinelli, Y. Filinchuk, Eur. J. Inorg. Chem. 2008, 3419–3422.
- 10
- 10aM. Cantuel, G. Bernardinelli, G. Muller, J. P. Riehl, C. Piguet, Inorg. Chem. 2004, 43, 1840–1849;
- 10bC. T. Yeung, W. T. Chan, S. C. Yan, K. L. Yu, K. H. Yim, W. T. Wong, G. L. Law, Chem. Commun. 2015, 51, 592–595;
- 10cX. Z. Li, L. P. Zhou, L. L. Yan, D. Q. Yuan, C. S. Lin, Q. F. Sun, J. Am. Chem. Soc. 2017, 139, 8237–8244.
- 11
- 11aJ. A. Kitchen, D. E. Barry, L. Mercs, M. Albrecht, R. D. Peacock, T. Gunnlaugsson, Angew. Chem. Int. Ed. 2012, 51, 704–708; Angew. Chem. 2012, 124, 728–732;
- 11bS. Petoud, J. G. Buznzli, T. Glanzman, C. Piguet, Q. Xiang, R. P. Thummel, J. Lumin. 1999, 82, 69–79.
- 12
- 12aF. Stomeo, C. Lincheneau, J. P. Leonard, J. E. O'Brien, R. D. Peacock, C. P. McCoy, T. Gunnlaugsson, J. Am. Chem. Soc. 2009, 131, 9636–9637;
- 12bT. Taniguchi, A. Tsubouchi, Y. Imai, J. Yuasa, H. Oguri, J. Org. Chem. 2018, 83, 15284–15296.
- 13
- 13aL. L. Yan, C. H. Tan, G. L. Zhang, L. P. Zhou, J. C. Bunzli, Q. F. Sun, J. Am. Chem. Soc. 2015, 137, 8550–8555;
- 13bC. T. Yeung, K. H. Yim, H. Y. Wong, R. Pal, W. S. Lo, S. C. Yan, M. Y.-M. Wong, D. Yufit, D. E. Smiles, L. J. McCormick, S. J. Teat, D. K. Shuh, W. T. Wong, G. L. Law, Nat. Commun. 2017, 8, 1128.
- 14
- 14aF. Renaud, C. Piguet, G. Bernardinelli, J. C. G. Buenzli, G. Hopfgartner, J. Am. Chem. Soc. 1999, 121, 9326–9342;
- 14bG. Zhang, G. Gil-Ramirez, A. Markevicius, C. Browne, I. J. Vitorica-Yrezabal, D. A. Leigh, J. Am. Chem. Soc. 2015, 137, 10437–10442.
- 15Z. Wang, L. He, B. Liu, L. P. Zhou, L. X. Cai, S. J. Hu, X. Z. Li, Z. Li, T. Chen, X. Li, Q. F. Sun, J. Am. Chem. Soc. 2020, 142, 16409–16419.
- 16G. J. Kleywegt, T. A. Jones, Acta Crystallogr. Sect. D 1994, 50, 178–185.
- 17A. L. Spek, J. Appl. Crystallogr. 2003, 36, 7–13.
- 18K. Nakanishi, N. Berova, R. W. Woody, Circular dichroism: principles and applications, 2nd ed., Wiley-VCH, New York, 2000.
- 19C. Tan, K. Yang, J. Dong, Y. Liu, Y. Liu, J. Jiang, Y. Cui, J. Am. Chem. Soc. 2019, 141, 17685–17695.
- 20Y. Tanaka, K. M.-C. Wong, V. W.-W. Yam, Chem. Eur. J. 2013, 19, 390–399.
- 21Deposition Number(s) 2039853, 2039854 and 2039855 (for 1-Eu, 2-Eu and 3-Eu) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.