Asymmetric Hydroacylation Involving Alkene Isomerization for the Construction of C3-Chirogenic Center
Chong Liu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontier Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorJing Yuan
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontier Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Dr. Zhenfeng Zhang
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorProf. Dr. Ilya D. Gridnev
Department of Chemistry, Graduate School of Science, Tohoku University, Aramaki 3–6, Aoba-ku, Sendai, 980-8578 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontier Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorChong Liu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontier Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorJing Yuan
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontier Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Dr. Zhenfeng Zhang
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorProf. Dr. Ilya D. Gridnev
Department of Chemistry, Graduate School of Science, Tohoku University, Aramaki 3–6, Aoba-ku, Sendai, 980-8578 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Wanbin Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontier Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
School of Pharmacy, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorGraphical Abstract
Abstract
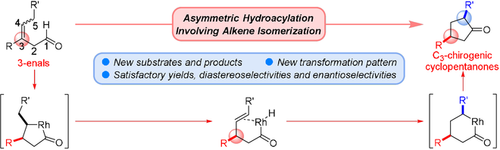
A new transformation pattern for enantioselective intramolecular hydroacylation has been developed involving an alkene isomerization strategy. Proceeding through a five-membered rhodacycle intermediate, 3-enals were converted to C3- or C3,C5-chirogenic cyclopentanones with satisfactory yields, diastereoselectivities, and enantioselectivities. A catalytic cycle has been theoretically calculated and the origin of the stereoselection is rationally explained.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202017190-sup-0001-misc_information.pdf13.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. I. Richardson, J. A. Dodge, G. L. Durst, L. A. Pfeifer, J. Shah, Y. Wang, J. D. Durbin, V. Krishnan, B. H. Norman, Bioorg. Med. Chem. Lett. 2007, 17, 4824;
- 1bS. A. Snyder, A. L. Zografos, Y. Lin, Angew. Chem. Int. Ed. 2007, 46, 8186; Angew. Chem. 2007, 119, 8334;
- 1cL. Liu, S. Liu, X. Chen, L. Guo, Y. Che, Bioorg. Med. Chem. 2009, 17, 606;
- 1dJ. Li, D. Zhang, X. Wu, Bioorg. Med. Chem. Lett. 2011, 21, 130;
- 1eA. Prandi, S. Franchini, L. I. Manasieva, P. Fossa, E. Cichero, G. Marucci, M. Buccioni, A. Cilia, L. Pirona, L. Brasili, J. Med. Chem. 2012, 55, 23;
- 1fM.-L. Tang, C. Zhong, Z.-Y. Liu, P. Peng, X.-H. Liu, X. Sun, Eur. J. Med. Chem. 2016, 113, 63;
- 1gJ. Yang, X.-M. Zhang, F.-M. Zhang, S.-H. Wang, Y.-Q. Tu, Z. Li, X. C. Wang, H. Wang, Angew. Chem. Int. Ed. 2020, 59, 8471; Angew. Chem. 2020, 132, 8549.
- 2For selected reviews on metal-catalyzed asymmetric hydroacylations, see:
- 2aM. C. Willis, Chem. Rev. 2010, 110, 725;
- 2bJ. C. Leung, M. J. Krische, Chem. Sci. 2012, 3, 2202;
- 2cS. K. Murphy, V. M. Dong, Chem. Commun. 2014, 50, 13645;
- 2dA. Ghosh, K. F. Johnson, K. L. Vickerman, J. A. Walker, L. M. Stanley, Org. Chem. Front. 2016, 3, 639.
- 3
- 3aB. R. James, C. G. Young, J. Chem. Soc. Chem. Commun. 1983, 1215;
- 3bB. R. James, C. G. Young, J. Organomet. Chem. 1985, 285, 321.
- 4For selected examples, see:
- 4aR. W. Barnhart, X. Wang, P. Noheda, S. H. Bergens, J. Whelan, B. Bosnich, J. Am. Chem. Soc. 1994, 116, 1821;
- 4bK. Kundu, J. V. McCullagh, A. T. Morehead, Jr., J. Am. Chem. Soc. 2005, 127, 16042;
- 4cT. J. Hoffman, E. M. Carreira, Angew. Chem. Int. Ed. 2011, 50, 10670; Angew. Chem. 2011, 123, 10858;
- 4dJ. Yang, N. Yoshikai, J. Am. Chem. Soc. 2014, 136, 16748;
- 4eA. Ghosh, L. M. Stanley, Chem. Commun. 2014, 50, 2765;
- 4fX.-W. Du, A. Ghosh, L. M. Stanley, Org. Lett. 2014, 16, 4036;
- 4gK. F. Johnson, A. C. Schmidt, L. M. Stanley, Org. Lett. 2015, 17, 4654;
- 4hE. J. Rastelli, N. T. Truong, D. M. Coltart, Org. Lett. 2016, 18, 5588;
- 4iK. F. Johnson, E. A. Schneider, B. P. Schumacher, A. Ellern, J. D. Scanlon, L. M. Stanley, Chem. Eur. J. 2016, 22, 15619;
- 4jK. L. Vickerman, L. M. Stanley, Org. Lett. 2017, 19, 5054;
- 4kJ. Yang, A. Rérat, Y. J. Lim, C. Gosmini, N. Yoshikai, Angew. Chem. Int. Ed. 2017, 56, 2449; Angew. Chem. 2017, 129, 2489;
- 4lZ. Chen, Y. Aota, H. M. H. Nguyen, V. M. Dong, Angew. Chem. Int. Ed. 2019, 58, 4705; Angew. Chem. 2019, 131, 4753.
- 5J.-W. Park, K. G. M. Kou, D. K. Kim, V. M. Dong, Chem. Sci. 2015, 6, 4479.
- 6The C3-chirogenic center has been obtained during the formation of C4-chirogenic center via a strategy of asymmetric desymmetrization:
- 6aX.-M. Wu, K. Funakoshi, K. Sakai, Tetrahedron Lett. 1993, 34, 5927;
- 6bM. Tanaka, M. Imai, M. Fujio, E. Sakamoto, M. Takahashi, Y. Eto-Kato, X. M. Wu, K. Funakoshi, K. Sakai, H. Suemune, J. Org. Chem. 2000, 65, 5806;
- 6cM. Tanaka, M. Takahashi, E. Sakamoto, M. Imai, A. Matsui, M. Fujio, K. Funakoshi, K. Sakai, H. Suemune, Tetrahedron 2001, 57, 1197; The example to construct only the C3-chirogenic center has been reported with poor enantioselectivity and yield via a strategy of kinetic resolution:
- 6dR. W. Barnhart, B. Bosnich, Organometallics 1995, 14, 4343.
- 7For selected examples of 5,4-migratory insertion of 4-enals, see:
- 7aJ.-W. Park, Z. Chen, V. M. Dong, J. Am. Chem. Soc. 2016, 138, 3310; For selected examples of C−C activation, see:
- 7bX. Zhou, H. M. Ko, G. Dong, Angew. Chem. Int. Ed. 2016, 55, 13867; Angew. Chem. 2016, 128, 14071;
- 7cL. Deng, T. Xu, H. Li, G. Dong, J. Am. Chem. Soc. 2016, 138, 369;
- 7dL. Deng, L. Jin, G. Dong, Angew. Chem. Int. Ed. 2018, 57, 2702; Angew. Chem. 2018, 130, 2732;
- 7eL. Deng, Y. Fu, S. Y. Lee, C. Wang, P. Liu, G. Dong, J. Am. Chem. Soc. 2019, 141, 16260.
- 8For review on alkene isomerization, see:
- 8aA. Vasseur, J. Bruffaerts, I. Marek, Nat. Chem. 2016, 8, 209; For selected examples of hydrogenations, see:
- 8bR. Wu, M. G. Beauchamps, J. M. Laquidara, J. R. Sowa, Jr., Angew. Chem. Int. Ed. 2012, 51, 2106; Angew. Chem. 2012, 124, 2148;
- 8cN. Arai, Y. Okabe, T. Ohkuma, Adv. Synth. Catal. 2019, 361, 5540; For selected examples of hydroborations, see:
- 8dA. J. Ruddy, O. L. Sydora, B. L. Small, M. Stradiotto, L. Turculet, Chem. Eur. J. 2014, 20, 13918;
- 8eM. L. Scheuermann, E. J. Johnson, P. J. Chirik, Org. Lett. 2015, 17, 2716;
- 8fX. Chen, Z. Cheng, J. Guo, Z. Lu, Nat. Commun. 2018, 9, 3939;
- 8gN. G. Léonard, W. N. Palmer, M. R. Friedfeld, M. J. Bezdek, P. J. Chirik, ACS Catal. 2019, 9, 9034; For selected examples of hydrosilylations, see:
- 8hS. Azpeitia, M. A. Garralda, M. A. Huertos, ChemCatChem 2017, 9, 1901;
- 8iS. Hanna, T. W. Butcher, J. F. Hartwig, Org. Lett. 2019, 21, 7129; For other examples of alkene isomerization, see:
- 8jC. Romano, C. Mazet, J. Am. Chem. Soc. 2018, 140, 4743;
- 8kX. Liu, W. Zhang, Y. Wang, Z.-X. Zhang, L. Jiao, Q. Liu, J. Am. Chem. Soc. 2018, 140, 6873;
- 8lW. Wang, C. Ding, Y. Li, Z. Li, Y. Li, L. Peng, G. Yin, Angew. Chem. Int. Ed. 2019, 58, 4612; Angew. Chem. 2019, 131, 4660;
- 8mY. He, C. Liu, L. Yu, S. Zhu, Angew. Chem. Int. Ed. 2020, 59, 9186; Angew. Chem. 2020, 132, 9271;
- 8nR. Yu, S. Rajasekar, X. Fang, Angew. Chem. Int. Ed. 2020, 59, 21436; Angew. Chem. 2020, 132, 21620.
- 9J. Yuan, C. Liu, Y. Chen, Z. Zhang, D. Yan, W. Zhang, Tetrahedron 2019, 75, 269.
- 10
- 10aJ. Jia, Z. Ling, Z. Zhang, K. Tamura, I. D. Gridnev, T. Imamoto, W. Zhang, Adv. Synth. Catal. 2018, 360, 738;
- 10bJ. Jia, D. Fan, J. Zhang, Z. Zhang, W. Zhang, Adv. Synth. Catal. 2018, 360, 3793.
- 11A plausible mechanism for the generation of byproduct 2 a′ via decarbonylation and alkene isomerization has been proposed in the Supporting Information, Scheme S1.
- 12I. D. Gridnev, ChemCatChem 2016, 8, 3463.