Organelle-Specific Photoactivation of DNA Nanosensors for Precise Profiling of Subcellular Enzymatic Activity
Yulei Shao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100149 China
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorDr. Jian Zhao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100149 China
Search for more papers by this authorProf. Jinying Yuan
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorProf. Yuliang Zhao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Prof. Lele Li
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100149 China
Search for more papers by this authorYulei Shao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100149 China
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorDr. Jian Zhao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100149 China
Search for more papers by this authorProf. Jinying Yuan
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorProf. Yuliang Zhao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
Search for more papers by this authorCorresponding Author
Prof. Lele Li
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100149 China
Search for more papers by this authorGraphical Abstract
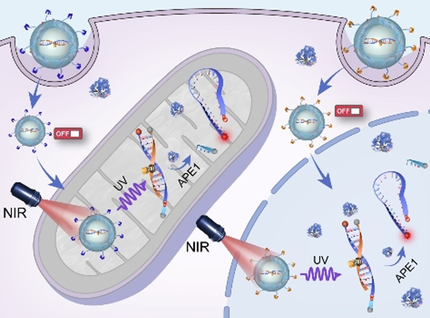
By integrating organelle-specific targeting and NIR-light-mediated photoactivation with DNA sensor technology, precise imaging of a specific enzyme in a chosen organelle (e.g., mitochondria or nucleus) was possible (see picture). The spatiotemporal control enabled imaging of enzyme translocation at subcellular resolution in response to oxidative stress in vitro and in vivo.
Abstract
Understanding of the functions of enzymes in diverse cellular processes is important, but the design of sensors with controllable localization for in situ imaging of subcellular levels of enzymatic activity is particularly challenging. We introduce herein a spatiotemporally controlled sensor technology that permits in situ localization and photoactivated imaging of human apurinic/apyrimidinic endonuclease 1 (APE1) within an intracellular organelle of choice (e.g., mitochondria or nucleus). The hybrid sensor platform is constructed by photoactivatable engineering of a DNA-based fluorescent probe and further combination with an upconversion nanoparticle and a specific organelle localization signal. Controlled localization and NIR-light-mediated photoactivation of the sensor “on demand” effectively constrains the imaging signal to the organelle of interest, with improved subcellular resolution. We further demonstrate the application of the nanosensors for the imaging of subcellular APE1 translocation in response to oxidative stress in live cells.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202016738-sup-0001-misc_information.pdf1.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. D. Scott, T. Pawson, Science 2009, 326, 1220–1224;
- 1bB. N. Kholodenko, J. F. Hancock, W. Kolch, Nat. Rev. Mol. Cell Biol. 2010, 11, 414–426.
- 2
- 2aC. D. Mol, T. Izumi, S. Mitra, J. Tainer, Nature 2000, 403, 451–456;
- 2bM. Li, D. M. Wilson, Antioxid. Redox Signaling 2014, 20, 678–707.
- 3
- 3aK. A. Robertson, H. A. Bullock, Y. Xu, R. Tritt, E. Zimmerman, T. M. Ulbright, R. S. Foster, L. H. Einhorn, M. R. Kelley, Cancer Res. 2001, 61, 2220–2225;
- 3bF. Shah, D. Logsdon, R. A. Messmann, J. C. Fehrenbacher, M. L. Fishel, M. R. Kelley, npj Precis. Oncol. 2017, 1, 19.
- 4D. Wang, D.-B. Xiang, X.-Q. Yang, L.-S. Chen, M.-X. Li, Z.-Y. Zhong, Y.-S. Zhang, Lung Cancer 2009, 66, 298–304.
- 5
- 5aL. Yuan, L. Wang, B. K. Agrawalla, S.-J. Park, H. Zhu, B. Sivaraman, J. Peng, Q.-H. Xu, Y.-T. Chang, J. Am. Chem. Soc. 2015, 137, 5930–5938;
- 5bJ. Huang, K. Pu, Angew. Chem. Int. Ed. 2020, 59, 11717–11731; Angew. Chem. 2020, 132, 11813–11827;
- 5cP. Cheng, Q. Miao, J. Li, J. G. Huang, C. Xie, K. Pu, J. Am. Chem. Soc. 2019, 141, 10581–10584;
- 5dH.-W. Rhee, P. Zou, N. D. Udeshi, J. D. Martell, V. K. Mootha, S. A. Carr, A. Y. Ting, Science 2013, 339, 1328–1331;
- 5eE. C. Greenwald, S. Mehta, J. Zhang, Chem. Rev. 2018, 118, 11707–11794.
- 6
- 6aR. Nutiu, Y. Li, J. Am. Chem. Soc. 2003, 125, 4771–4778;
- 6bS. Saha, V. Prakash, S. Halder, K. Chakraborty, Y. Krishnan, Nat. Nanotechnol. 2015, 10, 645–651;
- 6cD. Zheng, D. S. Seferos, D. A. Giljohann, P. C. Patel, C. A. Mirkin, Nano Lett. 2009, 9, 3258–3261;
- 6dZ. Tan, T. A. Feagin, J. M. Heemstra, J. Am. Chem. Soc. 2016, 138, 6328–6331;
- 6eA. E. Rangel, A. A. Hariri, M. Eisenstein, H. T. Soh, Adv. Mater. 2020, 32, 2003704;
- 6fI. A. P. Thompson, L. Zheng, M. Eisenstein, H. T. Soh, Nat. Commun. 2020, 11, 2946;
- 6gJ. Zhao, J. Gao, W. Xue, Z. Di, H. Xing, Y. Lu, L. Li, J. Am. Chem. Soc. 2018, 140, 578–581;
- 6hJ. S. Paige, T. Nguyen-Duc, W. Song, S. R. Jaffrey, Science 2012, 335, 1194;
- 6iG. S. Filonov, J. D. Moon, N. Svensen, S. R. Jaffrey, J. Am. Chem. Soc. 2014, 136, 16299–16308;
- 6jD. Samanta, S. B. Ebrahimi, C. D. Kusmierz, H. F. Cheng, C. A. Mirkin, J. Am. Chem. Soc. 2020, 142, 13350–13355.
- 7
- 7aZ. Wu, H. Fan, N. S. R. Satyavolu, W. Wang, R. Lake, J. Jiang, Y. Lu, Angew. Chem. Int. Ed. 2017, 56, 8721–8725; Angew. Chem. 2017, 129, 8847–8851;
- 7bH. Peng, X.-F. Li, H. Zhang, X. C. Le, Nat. Commun. 2017, 8, 14378;
- 7cL. Qiu, T. Zhang, J. Jiang, C. Wu, G. Zhu, M. You, X. Chen, L. Zhang, C. Cui, R. Yu, W. Tan, J. Am. Chem. Soc. 2014, 136, 13090–13093;
- 7dY. Lin, Z. Yang, R. J. Lake, C. Zheng, Y. Lu, Angew. Chem. Int. Ed. 2019, 58, 17061–17067; Angew. Chem. 2019, 131, 17217–17223;
- 7eS. B. Ebrahimi, D. Samanta, H. F. Cheng, L. I. Nathan, C. A. Mirkin, J. Am. Chem. Soc. 2019, 141, 13744–13748;
- 7fF. Chen, Q. Lu, L. Huang, B. Liu, M. Liu, Y. Zhang, J. Liu, Angew. Chem. Int. Ed. 2021, 60, 5453–5458; Angew. Chem. 2021, 133, 5513–5518.
- 8
- 8aW. E. Briley, M. H. Bondy, P. S. Randeria, T. J. Dupper, C. A. Mirkin, Proc. Natl. Acad. Sci. USA 2015, 112, 9591–9595;
- 8bZ. Wu, G. Q. Liu, X. L. Yang, J. H. Jiang, J. Am. Chem. Soc. 2015, 137, 6829–6836;
- 8cH. M. T. Choi, J. Y. Chang, L. A. Trinh, J. E. Padilla, S. E. Fraser, N. A. Pierce, Nat. Biotechnol. 2010, 28, 1208–1212;
- 8dZ. Cheglakov, T. M. Cronin, C. He, Y. Weizmann, J. Am. Chem. Soc. 2015, 137, 6116–6119;
- 8eJ. Hemphill, A. Deiters, J. Am. Chem. Soc. 2013, 135, 10512–10518;
- 8fZ. Qing, J. Xu, J. Hu, J. Zheng, L. He, Z. Zou, S. Yang, W. Tan, R. Yang, Angew. Chem. Int. Ed. 2019, 58, 11574–11585; Angew. Chem. 2019, 131, 11698–11709.
- 9
- 9aT.-T. Chen, X. Tian, C.-L. Liu, J. Ge, X. Chu, Y. Li, J. Am. Chem. Soc. 2015, 137, 982–989;
- 9bJ. Zhang, L. P. Smaga, N. S. R. Satyavolu, J. Chan, Y. Lu, J. Am. Chem. Soc. 2017, 139, 17225–17228;
- 9cJ. Zhai, Y. Liu, S. Huang, S. Fang, M. Zhao, Nucleic Acids Res. 2017, 45, e45–e45.
- 10
- 10aK. Dan, A. T. Veetil, K. Chakraborty, Y. Krishnan, Nat. Nanotechnol. 2019, 14, 252–259;
- 10bM. S. Jani, J. Zou, A. T. Veetil, Y. Krishnan, Nat. Chem. Biol. 2020, 16, 660–666.
- 11
- 11aK. Chakraborty, A. T. Veetil, S. R. Jaffrey, Y. Krishnan, Annu. Rev. Biochem. 2016, 85, 349–373;
- 11bS. B. Ebrahimi, D. Samanta, C. A. Mirkin, J. Am. Chem. Soc. 2020, 142, 11343–11356.
- 12
- 12aK. Deisseroth, Nat. Neurosci. 2015, 18, 1213–1225;
- 12bS. Chen, A. Z. Weitemier, X. Zeng, L. He, X. Wang, Y. Tao, A. J. Y. Huang, Y. Hashimotodani, M. Kano, H. Iwasaki, L. K. Parajuli, S. Okabe, D. B. L. Teh, A. H. All, I. Tsutsui-Kimura, K. F. Tanaka, X. Liu, T. J. McHugh, Science 2018, 359, 679;
- 12cZ. Yi, Z. Luo, X. Qin, Q. Chen, X. Liu, Acc. Chem. Res. 2020, 53, 2692–2704.
- 13
- 13aL. Kowalik, J. K. Chen, Nat. Chem. Biol. 2017, 13, 587–598;
- 13bA. Levskaya, O. D. Weiner, W. A. Lim, C. A. Voigt, Nature 2009, 461, 997–1001.
- 14
- 14aS. A. Reis, B. Ghosh, J. A. Hendricks, D. M. Szantai-Kis, L. Törk, K. N. Ross, J. Lamb, W. Read-Button, B. Zheng, H. Wang, C. Salthouse, S. J. Haggarty, R. Mazitschek, Nat. Chem. Biol. 2016, 12, 317–323;
- 14bY. Nihongaki, F. Kawano, T. Nakajima, M. Sato, Nat. Biotechnol. 2015, 33, 755–760.
- 15
- 15aJ. Zhao, H. Chu, Y. Zhao, Y. Lu, L. Li, J. Am. Chem. Soc. 2019, 141, 7056–7062;
- 15bH. Chu, J. Zhao, Y. Mi, Y. Zhao, L. Li, Angew. Chem. Int. Ed. 2019, 58, 14877–14881; Angew. Chem. 2019, 131, 15019–15023.
- 16
- 16aB. D. Freudenthal, W. A. Beard, M. J. Cuneo, N. S. Dyrkheeva, S. H. Wilson, Nat. Struct. Mol. Biol. 2015, 22, 924–931;
- 16bA. M. Whitaker, T. S. Flynn, B. D. Freudenthal, Nat. Commun. 2018, 9, 399.
- 17
- 17aF. Auzel, Chem. Rev. 2004, 104, 139–173;
- 17bH. Dong, S.-R. Du, X.-Y. Zheng, G.-M. Lyu, L.-D. Sun, L.-D. Li, P.-Z. Zhang, C. Zhang, C.-H. Yan, Chem. Rev. 2015, 115, 10725–10815;
- 17cY. Yang, Q. Shao, R. Deng, C. Wang, X. Teng, K. Cheng, Z. Cheng, L. Huang, Z. Liu, X. Liu, B. Xing, Angew. Chem. Int. Ed. 2012, 51, 3125–3129; Angew. Chem. 2012, 124, 3179–3183.
- 18
- 18aJ. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez, B. Kalyanaraman, Chem. Rev. 2017, 117, 10043–10120;
- 18bC. Liu, B. Liu, J. Zhao, Z. Di, D. Chen, Z. Gu, L. Li, Y. Zhao, Angew. Chem. Int. Ed. 2020, 59, 2634–2638; Angew. Chem. 2020, 132, 2656–2660.
- 19
- 19aH. J. Vaughan, J. J. Green, S. Y. Tzeng, Adv. Mater. 2020, 32, 1901081;
- 19bS. B. Hartono, W. Gu, F. Kleitz, J. Liu, L. He, A. P. J. Middelberg, C. Yu, G. Q. Lu, S. Z. Qiao, ACS Nano 2012, 6, 2104–2117.
- 20
- 20aA. G. Tkachenko, H. Xie, D. Coleman, W. Glomm, J. Ryan, M. F. Anderson, S. Franzen, D. L. Feldheim, J. Am. Chem. Soc. 2003, 125, 4700–4701;
- 20bS. S. Patel, B. J. Belmont, J. M. Sante, M. F. Rexach, Cell 2007, 129, 83–96.