Direct Catalytic Asymmetric Addition of Alkylnitriles to Aldehydes with Designed Nickel–Carbene Complexes
Dr. Akira Saito
Institute of Microbial Chemistry, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorDr. Shinya Adachi
Institute of Microbial Chemistry, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Dr. Naoya Kumagai
Institute of Microbial Chemistry, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masakatsu Shibasaki
Institute of Microbial Chemistry, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorDr. Akira Saito
Institute of Microbial Chemistry, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorDr. Shinya Adachi
Institute of Microbial Chemistry, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Dr. Naoya Kumagai
Institute of Microbial Chemistry, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masakatsu Shibasaki
Institute of Microbial Chemistry, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorGraphical Abstract
Abstract
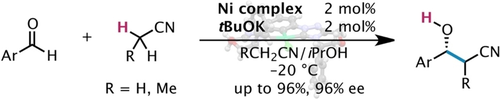
A direct catalytic asymmetric addition of acetonitrile to aldehydes that realizes over 90 % ee is the ultimate challenge in alkylnitrile addition chemistry. Herein, we report achieving high enantioselectivity by the strategic use of a sterically demanding NiII pincer carbene complex, which afforded highly enantioenriched β-hydroxynitriles. This highly atom-economical process paves the way for exploiting inexpensive acetonitrile as a promising C2 building block in a practical synthetic toolbox for asymmetric catalysis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202016690-sup-0001-misc_information.pdf5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. G. Vercade, P. B. Kisanga, Aldrichimica Acta 2004, 37, 3–14;
- 1bR. López, C. Palomo, Angew. Chem. Int. Ed. 2015, 54, 13170–13184; Angew. Chem. 2015, 127, 13366–13380.
- 2F. G. Bordwell, Acc. Chem. Res. 1988, 21, 456–463.
- 3R. Peters, S. Jautze, Synthesis 2009, 365–388.
- 4
- 4aM. B. Cid, S. Duce, S. Morales, E. Rodrigo, J. L. Ruano, Org. Lett. 2010, 12, 3586–3589;
- 4bR. Yazaki, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 5522–5531;
- 4cK. Hyodo, S. Nakamura, K. Tsuji, T. Ogawa, Y. Funahashi, N. Shibata, Adv. Synth. Catal. 2011, 353, 3385–3390;
- 4dY. Yanagida, R. Yazaki, N. Kumagai, M. Shibasaki, Angew. Chem. Int. Ed. 2011, 50, 7910–7914; Angew. Chem. 2011, 123, 8056–8060;
- 4eM. Kondo, M. Omori, T. Hatanaka, Y. Funahashi, S. Nakamura, Angew. Chem. Int. Ed. 2017, 56, 8677–8680; Angew. Chem. 2017, 129, 8803–8806.
- 5
- 5aM. Kondo, M. Sugimoto, S. Nakamura, Chem. Commun. 2016, 52, 13604–13607;
- 5bP. V. Balaji, L. Brewitz, N. Kumagai, M. Shibasaki, Angew. Chem. Int. Ed. 2019, 58, 2644–2648; Angew. Chem. 2019, 131, 2670–2674;
- 5cR. Ding, Z. A. De Los Santos, C. Wolf, ACS Catal. 2019, 9, 2169–2176;
- 5dP. V. Balaji, Z. Li, A. Saito, N. Kumagai, M. Shibasaki, Chem. Eur. J. 2020, 26, 15524–15527.
- 6
- 6aM. Kondo, N. Kobayashi, T. Hatanaka, Y. Funahashi, S. Nakamura, Chem. Eur. J. 2015, 21, 9066–9070;
- 6bM. Kondo, H. Saito, S. Nakamura, Chem. Commun. 2017, 53, 6776–6779;
- 6cS. Nakamura, A. Tokunaga, H. Saito, M. Kondo, Chem. Commun. 2019, 55, 5391–5394.
- 7
- 7aT. Arai, I. Oka, T. Morihata, A. Awata, H. Masu, Chem. Eur. J. 2013, 19, 1554–1557;
- 7bX. Chen, H. Chen, X. Ji, H. Jiang, Z. J. Yao, H. Liu, Org. Lett. 2013, 15, 1846–1849;
- 7cS. Kuwano, T. Suzuki, Y. Hosaka, T. Arai, Chem. Commun. 2018, 54, 3847–3850;
- 7dT. Arai, A. Nakamura, Synlett 2019, 30, 1356–1360.
- 8
- 8aB. A. D'Sa, P. Kisanga, J. G. Verkade, J. Org. Chem. 1998, 63, 3961–3967;
- 8bP. Kisanga, D. McLeod, B. D'Sa, J. Verkade, J. Org. Chem. 1999, 64, 3090–3094;
- 8cP. B. Kisanga, J. G. Verkade, J. Org. Chem. 2000, 65, 5431–5432;
- 8dJ. G. Verkade, P. B. Kisanga, Tetrahedron 2003, 59, 7819–7858;
- 8eN. Kumagai, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2004, 126, 13632–13633;
- 8fL. Fan, O. V. Ozerov, Chem. Commun. 2005, 4450–4452;
- 8gN. Kumagai, S. Matsunaga, M. Shibasaki, Chem. Commun. 2005, 3600–3602;
- 8hN. Kumagai, S. Matsunaga, M. Shibasaki, Tetrahedron 2007, 63, 8598–8608;
- 8iA. Goto, K. Endo, Y. Ukai, S. Irle, S. Saito, Chem. Commun. 2008, 2212–2214;
- 8jA. Goto, H. Naka, R. Noyori, S. Saito, Chem. Asian J. 2011, 6, 1740–1743;
- 8kS. Chakraborty, Y. J. Patel, J. A. Krause, H. Guan, Angew. Chem. Int. Ed. 2013, 52, 7523–7526; Angew. Chem. 2013, 125, 7671–7674;
- 8lA. Ariafard, H. Ghari, Y. Khaledi, A. Hossein Bagi, T. S. Wierenga, M. G. Gardiner, A. J. Canty, ACS Catal. 2016, 6, 60–68.
- 9
- 9aY. Suto, N. Kumagai, S. Matsunaga, M. Kanai, M. Shibasaki, Org. Lett. 2003, 5, 3147–3150;
- 9bY. Suto, R. Tsuji, M. Kanai, M. Shibasaki, Org. Lett. 2005, 7, 3757–3760;
- 9cY. Kawato, N. Kumagai, M. Shibasaki, Chem. Commun. 2013, 49, 11227–11229;
- 9dT. Deng, H. Wang, C. Cai, Eur. J. Org. Chem. 2014, 7259–7264;
- 9eD. Sureshkumar, V. Ganesh, N. Kumagai, M. Shibasaki, Chem. Eur. J. 2014, 20, 15723–15726;
- 9fY. Yamashita, I. Sato, H. Suzuki, S. Kobayashi, Chem. Asian J. 2015, 10, 2143–2146;
- 9gS. Lin, N. Kumagai, M. Shibasaki, Org. Biomol. Chem. 2016, 14, 9725–9730;
- 9hA. Saito, N. Kumagai, M. Shibasaki, Org. Lett. 2019, 21, 8187–8190.
- 10S. E. Denmark, T. W. Wilson, M. T. Burk, J. R. Heemstra, Jr., J. Am. Chem. Soc. 2007, 129, 14864–14865.
- 11S. M. Weinreb, R. K. Orr, Synthesis 2005, 1205–1227.
- 12Y. Ma, S. Wei, J. Wu, F. Yang, B. Liu, J. Lan, S. Yang, J. You, Adv. Synth. Catal. 2008, 350, 2645–2651.
- 13S. Wei, X. Li, Z. Yang, J. Lan, G. Gao, Y. Xue, J. You, Chem. Sci. 2012, 3, 359–363.
- 14
- 14aA. Poater, F. Ragone, R. Mariz, R. Dorta, L. Cavallo, Chem. Eur. J. 2010, 16, 14348–14353;
- 14bL. Falivene, R. Credendino, A. Poater, A. Petta, L. Serra, R. Oliva, V. Scarano, L. Cavallo, Organometallics 2016, 35, 2286–2293;
- 14cL. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano, L. Cavallo, Nat. Chem. 2019, 11, 872–879.
- 15The crystallographic data is associated with Ref. [8h].
- 16The reaction using aliphatic aldehyde needs further development: the reaction of acetonitrile and cyclohexanecarboxaldehyde gave the corresponding product in 14 % yield (potassium salt of BHT was used instead of tBuOK, rt, 24 h).
- 17The reaction using ketone needs to be further development: methyl benzoylformate gave the corresponding product in 45 % yield and 45 % ee (w/o iPrOH, 0 °C, 24 h).