Chiral Near-Infrared Fluorophores by Self-Promoted Oxidative Coupling of Cationic Helicenes with Amines/Enamines
Corresponding Author
Dr. Johann Bosson
Department of Organic Chemistry, University of Geneva, Quai Ernest Ansermet 30, 1211 Geneva 4, Switzerland
Search for more papers by this authorGeraldine M. Labrador
Department of Organic Chemistry, University of Geneva, Quai Ernest Ansermet 30, 1211 Geneva 4, Switzerland
Search for more papers by this authorDr. Céline Besnard
Laboratoire de Cristallographie, University of Geneva, Quai Ernest Ansermet 24, 1211 Geneva 4, Switzerland
Search for more papers by this authorCorresponding Author
Prof. Denis Jacquemin
CEISAM UMR 6230, CNRS, University of Nantes, 44000 Nantes, France
Search for more papers by this authorCorresponding Author
Prof. Jérôme Lacour
Department of Organic Chemistry, University of Geneva, Quai Ernest Ansermet 30, 1211 Geneva 4, Switzerland
Search for more papers by this authorCorresponding Author
Dr. Johann Bosson
Department of Organic Chemistry, University of Geneva, Quai Ernest Ansermet 30, 1211 Geneva 4, Switzerland
Search for more papers by this authorGeraldine M. Labrador
Department of Organic Chemistry, University of Geneva, Quai Ernest Ansermet 30, 1211 Geneva 4, Switzerland
Search for more papers by this authorDr. Céline Besnard
Laboratoire de Cristallographie, University of Geneva, Quai Ernest Ansermet 24, 1211 Geneva 4, Switzerland
Search for more papers by this authorCorresponding Author
Prof. Denis Jacquemin
CEISAM UMR 6230, CNRS, University of Nantes, 44000 Nantes, France
Search for more papers by this authorCorresponding Author
Prof. Jérôme Lacour
Department of Organic Chemistry, University of Geneva, Quai Ernest Ansermet 30, 1211 Geneva 4, Switzerland
Search for more papers by this authorGraphical Abstract
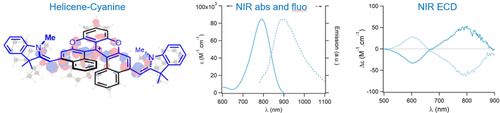
Cationic dioxa[6]helicene oxidizes alkyl amines to enamines and further reacts in situ as an electrophile to form, in one-pot multi-step cascades, fully delocalized mono or bis donor-π-acceptor coupling products. This original approach provides chiral cyanine-like chromophores that absorb up to the NIR and fluoresce alike, with intense NIR ECD (Δϵ up to 60 M−1 cm−1). DFT showcases the importance of conformers in the strong chiroptical response.
Abstract
In one pot, tertiary alkyl amines are oxidized to enamines by cationic dioxa[6]helicene, which further reacts as electrophile and oxidant to form mono or bis donor-π-acceptor coupling products. This original and convergent synthetic approach provides a strong extension of conjugation yielding chromophores that absorb intensively in far-red or NIR domains (λmax up to 791 nm) and fluoresce in the NIR as well (λmax up to 887 nm). Intense ECD properties around 790 nm with a |Δϵ| value up to 60 M−1 cm−1 are observed.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202016643-sup-0001-misc_information.pdf4.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Gingras, Chem. Soc. Rev. 2013, 42, 1051–1095;
- 1bP. Aillard, A. Voituriez, A. Marinetti, Dalton Trans. 2014, 43, 15263–15278;
- 1cJ. Bosson, J. Gouin, J. Lacour, Chem. Soc. Rev. 2014, 43, 2824–2840;
- 1dN. Saleh, C. Shen, J. Crassous, Chem. Sci. 2014, 5, 3680–3694;
- 1eH. Isla, J. Crassous, C. R. Chim. 2016, 19, 39–49;
- 1fM. Shigeno, Y. Kushida, M. Yamaguchi, Chem. Commun. 2016, 52, 4955–4970;
- 1gC.-F. Chen, Y. Shen, Helicene Chemistry From Synthesis to Applications, Springer, Berlin, Heidelberg, 2017;
10.1007/978-3-662-53168-6 Google Scholar
- 1hI. Stary, I. G. Stara, Targets Heterocycl. Syst. 2017, 21, 23–53;
- 1iK. Dhbaibi, L. Favereau, J. Crassous, Chem. Rev. 2019, 119, 8846–8953;
- 1jK. Kato, Y. Segawa, K. Itami, Synlett 2019, 30, 370–377;
- 1kW.-L. Zhao, M. Li, H.-Y. Lu, C.-F. Chen, Chem. Commun. 2019, 55, 13793–13803.
- 2E. Vander Donckt, J. Nasielski, J. R. Greenleaf, J. B. Birks, Chem. Phys. Lett. 1968, 2, 409–410.
- 3
- 3aJ. OuYang, J. Crassous, Coord. Chem. Rev. 2018, 376, 533–547;
- 3bE. S. Gauthier, R. Rodriguez, J. Crassous, Angew. Chem. Int. Ed. 2020, 59, 22840–22856; Angew. Chem. 2020, 132, 23036–23052.
- 4
- 4aM. Li, Y. Niu, X. Zhu, Q. Peng, H.-Y. Lu, A. Xia, C.-F. Chen, Chem. Commun. 2014, 50, 2993–2995;
- 4bM. Li, W. Yao, J.-D. Chen, H.-Y. Lu, Y. Zhao, C.-F. Chen, J. Mater. Chem. C 2014, 2, 8373–8380;
- 4cM. Li, H.-Y. Lu, C. Zhang, L. Shi, Z. Tang, C.-F. Chen, Chem. Commun. 2016, 52, 9921–9924;
- 4dZ.-H. Zhao, X. Liang, M.-X. He, M.-Y. Zhang, C.-H. Zhao, Org. Lett. 2019, 21, 9569–9573;
- 4eS. K. Pedersen, K. Eriksen, M. Pittelkow, Angew. Chem. Int. Ed. 2019, 58, 18419–18423; Angew. Chem. 2019, 131, 18590–18594;
- 4fK. Dhbaibi, L. Favereau, M. Srebro-Hooper, C. Quinton, N. Vanthuyne, L. Arrico, T. Roisnel, B. Jamoussi, C. Poriel, C. Cabanetos, J. Autschbach, J. Crassous, Chem. Sci. 2020, 11, 567–576.
- 5
- 5aF. Torricelli, J. Bosson, C. Besnard, M. Chekini, T. Bürgi, J. Lacour, Angew. Chem. Int. Ed. 2013, 52, 1796–1800; Angew. Chem. 2013, 125, 1840–1844;
- 5bJ. Bosson, G. M. Labrador, S. Pascal, F.-A. Miannay, O. Yushchenko, H. Li, L. Bouffier, N. Sojic, R. C. Tovar, G. Muller, D. Jacquemin, A. D. Laurent, B. Le Guennic, E. Vauthey, J. Lacour, Chem. Eur. J. 2016, 22, 18394–18403;
- 5cI. H. Delgado, S. Pascal, A. Wallabregue, R. Duwald, C. Besnard, L. Guenee, C. Nancoz, E. Vauthey, R. C. Tovar, J. L. Lunkley, G. Muller, J. Lacour, Chem. Sci. 2016, 7, 4685–4693;
- 5dR. Duwald, S. Pascal, J. Bosson, S. Grass, C. Besnard, T. Buergi, J. Lacour, Chem. Eur. J. 2017, 23, 13596–13601;
- 5eR. Duwald, J. Bosson, S. Pascal, S. Grass, F. Zinna, C. Besnard, L. Di Bari, D. Jacquemin, J. Lacour, Chem. Sci. 2020, 11, 1165–1169.
- 6S. Wang, B. Li, F. Zhang, ACS Cent. Sci. 2020, 6, 1302–1316.
- 7
- 7aR. Vanel, F.-A. Miannay, E. Vauthey, J. Lacour, Chem. Commun. 2014, 50, 12169–12172;
- 7bD. Wang, X. Guo, H. Wu, Q. Wu, H. Wang, X. Zhang, E. Hao, L. Jiao, J. Org. Chem. 2020, 85, 8360–8370.
- 8G. M. Labrador, C. Besnard, T. Burgi, A. I. Poblador-Bahamonde, J. Bosson, J. Lacour, Chem. Sci. 2019, 10, 7059–7067.
- 9As compound 1 reacts twice as an oxidant and only once as an electrophile, the maximum isolated yield is 33 % only.
- 10G. M. Labrador, J. Bosson, Z. S. Breitbach, Y. Lim, E. R. Francotte, R. Sabia, C. Villani, D. W. Armstrong, J. Lacour, Chirality 2016, 28, 282–289.
- 11
- 11aR. R. Fraser, R. B. Swingle, Tetrahedron 1969, 25, 3469–3475;
- 11bS. L. Schreiber, Tetrahedron Lett. 1980, 21, 1027–1030;
- 11cJ. J. Talley, Tetrahedron Lett. 1981, 22, 823–826.
- 12
- 12aZ. H. Yao, Q. Zhou, J. Org. Chem. 1987, 52, 3552–3558;
- 12bA. Morigaki, M. Kawamura, S. Arimitsu, T. Ishihara, T. Konno, Asian J. Org. Chem. 2013, 2, 239–243.
- 13X.-F. Xia, X.-Z. Shu, K.-G. Ji, Y.-F. Yang, A. Shaukat, X.-Y. Liu, Y.-M. Liang, J. Org. Chem. 2010, 75, 2893–2902.
- 14N. Takasu, K. Oisaki, M. Kanai, Org. Lett. 2013, 15, 1918–1921.
- 15M.-J. Zhou, S.-F. Zhu, Q.-L. Zhou, Chem. Commun. 2017, 53, 8770–8773.
- 16R. J. Griffiths, W. C. Kong, S. A. Richards, G. A. Burley, M. C. Willis, E. P. A. Talbot, Chem. Sci. 2018, 9, 2295–2300.
- 17
- 17aK. Kiyokawa, T. Kosaka, T. Kojima, S. Minakata, Angew. Chem. Int. Ed. 2015, 54, 13719–13723; Angew. Chem. 2015, 127, 13923–13927;
- 17bH. Jiang, X. Tang, Z. Xu, H. Wang, K. Han, X. Yang, Y. Zhou, Y.-L. Feng, X.-Y. Yu, Q. Gui, Org. Biomol. Chem. 2019, 17, 2715–2720.
- 18J. Choi, A. H. R. MacArthur, M. Brookhart, A. S. Goldman, Chem. Rev. 2011, 111, 1761–1779.
- 19Enamines are also generated in photoredox processes using trialkylamines as sacrificial electron donors, see
- 19aA. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075–10166;
- 19bJ.-R. Chen, X.-Q. Hu, L.-Q. Lu, W.-J. Xiao, Chem. Soc. Rev. 2016, 45, 2044–2056;
- 19cJ. Hu, J. Wang, T. H. Nguyen, N. Zheng, Beilstein J. Org. Chem. 2013, 9, 1977–2001.
- 20S. Yokoyama, T. Hirose, K. Matsuda, Chem. Lett. 2015, 44, 76–78.
- 21Using the Kekule representation of 2 in Scheme 1, the s-cis conformation is the only one observed in the 1H NMR spectra at room temperature. This analysis holds for 3 and 4.
- 22Hydride abstraction by the benzophenone in an Oppenauer-like oxidation has been mechanistically considered even if benzhydrol could not be detected in the reaction mixture.
- 23Under these conditions, recovery of leuco adduct 7, or its oxidation to starting material 1 after reaction completion, could not be achieved readily.
- 24This flattening of the helicenes in the solid state may be due to intermolecular packing interactions.
- 25
- 25aH. Li, A. Wallabregue, C. Adam, G. M. Labrador, J. Bosson, L. Bouffier, J. Lacour, N. Sojic, J. Phys. Chem. C 2017, 121, 785–792;
- 25bH. Li, S. Voci, A. Wallabregue, C. Adam, G. M. Labrador, R. Duwald, I. Hernández Delgado, S. Pascal, J. Bosson, J. Lacour, L. Bouffier, N. Sojic, ChemElectroChem 2017, 4, 1750–1756.
- 26A second mono electronic non-reversible reduction leading to the neutral helicene happens at −1.08 V vs. Ag.
- 27H. Mustroph, A. Towns, ChemPhysChem 2018, 19, 1016–1023.
- 28A. V. Kulinich, E. K. Mikitenko, A. A. Ishchenko, Phys. Chem. Chem. Phys. 2016, 18, 3444–3453.
- 29P. Sun, C. Wang, Z. S. Breitbach, Y. Zhang, D. W. Armstrong, Anal. Chem. 2009, 81, 10215–10226.
- 30Cationic[6]helicenes are highly configurationally stable derivatives presenting racemization barriers from 29.8 to >37 kcal mol−1. In compounds 2–4, the added electron-donor group(s) likely increase the configurational stability of the derivatives in addition. See reference [10].
- 31Y. Nakai, T. Mori, Y. Inoue, J. Phys. Chem. A 2013, 117, 83–93.
- 32Preliminary CPL measurement was attempted with (−)-2 and (+)-2. Unfortunately, the signal was below detection limits (glum<10−4), in line with the relatively weak gabs value (7×10−4). See H. Tanaka, Y. Inoue, T. Mori, ChemPhotoChem 2018, 2, 386–402.
- 33T. Mori, Circularly Polarized Luminescence of Isolated Small Organic Molecules, Springer, Singapore, 2020.
10.1007/978-981-15-2309-0 Google Scholar
- 34Deposition Numbers 2050126 (for 3) and 2050127 (for 4) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures. In addition, the dataset for this article can be found at the following https://doi.org/10.26037/yareta:6ar3rijxhfeclcold2y2mcsbhm.