Prebiotic Peptide Synthesis and Spontaneous Amyloid Formation Inside a Proto-Cellular Compartment
Graphical Abstract
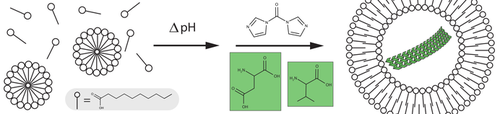
Self-organization in a system of fatty acids and activated amino acids leads to vesicle-encapsulated amyloids. The fatty-acid vesicles act both as a filter, allowing the selective passage of activated amino acids, and as a barrier, blocking the diffusion of the amyloidogenic peptides that form spontaneously inside the vesicles. Hence, a simple mixture of prebiotically plausible starting materials leads to organizationally complex structures.
Abstract
Cellular life requires a high degree of molecular complexity and self-organization, some of which must have originated in a prebiotic context. Here, we demonstrate how both of these features can emerge in a plausibly prebiotic system. We found that chemical gradients in simple mixtures of activated amino acids and fatty acids can lead to the formation of amyloid-like peptide fibrils that are localized inside of a proto-cellular compartment. In this process, the fatty acid or lipid vesicles act both as a filter, allowing the selective passage of activated amino acids, and as a barrier, blocking the diffusion of the amyloidogenic peptides that form spontaneously inside the vesicles. This synergy between two distinct building blocks of life induces a significant increase in molecular complexity and spatial order thereby providing a route for the early molecular evolution that could give rise to a living cell.
Conflict of interest
The authors declare no conflict of interest.