Unified Approach to Furan Natural Products via Phosphine-Palladium Catalysis
Violet Yijang Chen
Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA, 90095-1659 USA
Search for more papers by this authorCorresponding Author
Ohyun Kwon
Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA, 90095-1659 USA
Search for more papers by this authorViolet Yijang Chen
Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA, 90095-1659 USA
Search for more papers by this authorCorresponding Author
Ohyun Kwon
Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA, 90095-1659 USA
Search for more papers by this authorGraphical Abstract
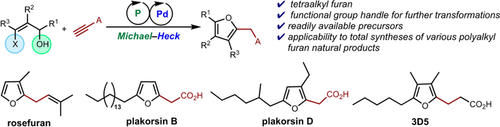
Highly substituted polyalkyl furans can be synthesized using sequential Michael–Heck reactions between functionalized (Z)-β-halo allylic alcohols and activated alkynes. This method provides facile access to tetraalkyl furans containing a C2-carboxyalkyl chain, which is readily functionalized to enable the total syntheses of several classes of bioactive furans, including furanoterpenes, polyketides, and furan fatty acids.
Abstract
Polyalkyl furans are widespread in nature, often performing important biological roles. Despite a plethora of methods for the synthesis of tetrasubstituted furans, the construction of tetraalkyl furans remains non-trivial. The prevalence of alkyl groups in bioactive furan natural products, combined with the desirable bioactivities of tetraalkyl furans, calls for a general synthetic protocol for polyalkyl furans. This paper describes a Michael–Heck approach, using sequential phosphine-palladium catalysis, for the preparation of various polyalkyl furans from readily available precursors. The versatility of this method is illustrated by the total syntheses of nine distinct polyalkylated furan natural products belonging to different classes, namely the furanoterpenes rosefuran, sesquirosefuran, and mikanifuran; the marine natural products plakorsins A, B, and D and plakorsin D methyl ester; and the furan fatty acids 3D5 and hydromumiamicin.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202015232-sup-0001-misc_information.pdf15.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Ochi, K. Yamada, H. Kawakami, A. Tatsukawa, H. Kotsuki, K. Shibata, Tetrahedron Lett. 1992, 33, 7531–7534;
- 1bA. Boto, L. Ivarez, Heterocycles in Natural Products Synthesis (Eds.: K. C. Majumdar, S. K. Chattopadhyay), Wiley-VCH, Weinheim, 2011, pp. 97–152;
10.1002/9783527634880.ch4 Google Scholar
- 1cA. R. Katritzky, Advanced Heterocyclic Chemistry, Academic Press, New York, 1982;
- 1dR. A. Craig, B. M. Stoltz, Chem. Rev. 2017, 117, 7878–7909.
- 2
- 2aA. F. Pozharskii, A. T. Soldatenkov, A. R. Katritzky, Heterocycles in Life and Society, Wiley, Chichester, 1997;
- 2bJ. B. Sperry, D. L. Wright, Curr. Opin. Drug Discovery Dev. 2011, 8, 723–740;
- 2cR. Banerjee, H. Kumar, M. Banerjee, Int. J. Rev. Life Sci. 2012, 2, 7–16.
- 3
- 3aS. F. Martin, D. E. Guinn, Tetrahedron Lett. 1984, 25, 5607–5610;
- 3bB. H. Lipshutz, Chem. Rev. 1986, 86, 795–819.
- 4
- 4aR. J. Bartelt, A. A. Cosse, B. W. Zilkowski, D. Weisleder, S. H. Grode, R. N. Wiedenmann, S. L. Post, J. Chem. Ecol. 2006, 32, 693–712;
- 4bR. J. Petroski, R. J. Bartelt, K. Vermillion, Synth. Commun. 2009, 39, 1389–1405.
- 5
- 5aG. Spiteller, Lipids 2005, 40, 755–771;
- 5bR. A. S. Lemke, A. C. Peterson, E. C. Ziegelhoffer, M. S. Westphall, H. Tjellstrom, J. J. Coon, T. J. Donohue, Proc. Natl. Acad. Sci. USA 2014, 111, 3450–3457;
- 5cL. Xu, A. J. Sinclair, M. Faiza, D. Li, X. Han, H. Yin, Y. Wang, Prog. Lipid Res. 2017, 68, 119–137;
- 5dT. Wakimoto, H. Kondo, H. Nii, K. Kimura, Y. Egami, Y. Oka, M. Yoshida, E. Kida, Y. Ye, S. Akahoshi, T. Asakawa, K. Matsumura, H. Ishida, H. Nukaya, K. Tsuji, T. Kan, I. Abe, Proc. Natl. Acad. Sci. USA 2011, 108, 17533–17537.
- 6
- 6aP. Weyerstahl, A. Schenk, H. Marschall, Liebigs Ann. 1995, 1849–1853;
- 6bN. Mori, Y. Kuwahara, K. Kurosa, J. Chem. Ecol. 1998, 24, 1771–1779;
- 6cN. Mori, Y. Kuwahara, J. Chem. Ecol. 2000, 26, 1299–1309.
- 7
- 7aJ. Zhang, X. Tang, J. Li, P. Li, N. J. de Voogd, X. Ni, X. Jin, X. Yao, P. Li, G. Li, J. Nat. Prod. 2013, 76, 600–606;
- 7bG. Chianese, H.-B. Yu, F. Yang, C. Sirignano, P. Luciano, B.-N. Han, S. Khan, H.-W. Lin, O. Taglialatela-Scafati, J. Org. Chem. 2016, 81, 5135–5143.
- 8
- 8aS. Al-Busafi, R. C. Whitehead, Tetrahedron Lett. 2000, 41, 3467–3470;
- 8bY. C. Shen, C. V. Prakash, Y. H. Kuo, J. Nat. Prod. 2001, 64, 324–327;
- 8cS. Al-Busafi, J. R. Doncaster, M. G. B. Drew, A. C. Regan, R. C. Whitehead, J. Chem. Soc. Perkin Trans. 1 2002, 476–484;
- 8dL. L. Etchells, A. Sardarian, R. C. Whitehead, Tetrahedron Lett. 2005, 46, 2803–2807;
- 8eJ. R. Doncaster, L. L. Etchells, N. M. Kershaw, R. Nakamura, H. Ryan, R. Takeuchi, K. Sakaguchi, A. Sardiarian, R. C. Whitehead, Bioorg. Med. Chem. Lett. 2006, 16, 2877–2881.
- 9
- 9aY. Kojima, S. Wakita, N. Kato, Tetrahedron Lett. 1979, 20, 4577–4580;
10.1016/S0040-4039(01)86653-3 Google Scholar
- 9bS. P. Tanis, P. M. Herrinton, J. Org. Chem. 1983, 48, 4572–4580.
- 10Selected examples:
- 10aT. T. Kao, S. Syu, Y. W. Jhang, W. Lin, Org. Lett. 2010, 12, 3066–3069;
- 10bC. Raji Reddy, M. D. Reddy, J. Org. Chem. 2014, 79, 106–116;
- 10cY. Y. Han, Y. Y. Jiao, D. Ren, Z. Hu, S. Shen, S. Yu, Asian J. Org. Chem. 2017, 6, 414–417;
- 10dY. Yuan, H. Tan, L. Kong, Z. Zheng, M. Xu, J. Huang, Y. Li, Org. Biomol. Chem. 2019, 17, 2725–2733.
- 11Selected examples:
- 11aJ. Lou, Q. Wang, K. Wu, P. Wu, Z. Yu, Org. Lett. 2017, 19, 3287–3290;
- 11bT. Ryu, D. Eom, S. Shin, J. U. Son, P. H. Lee, Org. Lett. 2017, 19, 452–455;
- 11cQ. Wang, Z. Liu, J. Lou, Z. Yu, Org. Lett. 2018, 20, 6007–6011;
- 11dS. J. Gharpure, P. V. Prasath, Y. G. Shelke, Org. Lett. 2019, 21, 223–227.
- 12Selected examples:
- 12aA. Fayol, J. Zhu, Org. Lett. 2004, 6, 115–118;
- 12bC. Cheng, S. Liu, G. Zhu, Org. Lett. 2015, 17, 1581–1584;
- 12cA. L. Siva Kumari, K. C. K. Swamy, J. Org. Chem. 2016, 81, 1425–1433;
- 12dH. Cai, R. S. Thombal, X. Li, Y. R. Lee, Adv. Synth. Catal. 2019, 361, 4022–4032.
- 13Selected examples:
- 13aA. D. Dudnik, A. W. Sromek, M. Rubina, J. T. Kim, A. V. Kel'I, V. Gevorgyan, J. Am. Chem. Soc. 2008, 130, 1440–1452;
- 13bN. Okamoto, R. Yanada, J. Org. Chem. 2012, 77, 3944–4951;
- 13cC. H. Cho, F. Shi, D. I. Jung, B. Neuenswander, G. H. Lushington, R. C. Larock, ACS Comb. Sci. 2012, 14, 403–414;
- 13dR. N. Ram, D. K. Gupta, V. K. Soni, J. Org. Chem. 2016, 81, 1665–1674;
- 13eA. Kondoh, K. Aita, S. Ishiwaka, M. Terada, Org. Lett. 2020, 22, 2105–2110.
- 14To the best of our knowledge, the following reports are the only ones describing the syntheses of tetraalkyl furans:
- 14aZ. Chai, Z. F. Xie, X. Y. Liu, G. Zhao, J. D. Wang, J. Org. Chem. 2008, 73, 2947–2950;
- 14bN. M. Chobanov, M. G. Shaibakova, N. R. Popod'ko, L. O. Khafizova, U. M. Dzhemilev, Tetrahedron 2017, 73, 5639–5645;
- 14cL. O. Khafizova, N. M. Chobanov, M. G. Shaibakova, N. R. Popod'ko, T. V. Tyumkina, U. M. Dzhemilev, Tetrahedron 2018, 74, 2482–2487; see the Supporting Information for a list of the syntheses of 90 tetrasubstituted furans.
- 15For examples of aryl-containing furan natural products, furoguaiacidin diethyl ether, shikonofurans A–E, and roseophilin, see:
- 15aF. G. Schreiber, R. Stevenson, J. Org. Chem. 1975, 40, 386–387;
- 15bF. Yoshizaki, S. Hisamichi, Y. Kondo, Y. Sato, S. Nozoe, Chem. Pharm. Bull. 1982, 30, 4407–4411;
- 15cJ. H. Frederich, P. G. Harran, J. Am. Chem. Soc. 2013, 135, 3788–3791.
- 16Synthesis of (Z)-3-iodo-2-propen-1-ols:
- 16aJ. G. Duboudin, B. Jousseaume, A. Bonakdar, A. Saux, J. Organomet. Chem. 1979, 168, 227–232;
- 16bA. Cowell, J. K. Stille, J. Am. Chem. Soc. 1980, 102, 4193–4198.
- 17
- 17aJ. Inanaga, Y. Baba, T. Hanamoto, Chem. Lett. 1993, 22, 241–244;
- 17bA. Ramazani, B. P. Pakravan, M. Bandpey, N. Noshiranzadeh, A. Souldozi, Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1633–1640;
- 17cD. Tejedor, S. J. Alvarez-Mendez, J. M. Lopez-Soria, V. S. Martin, F. Garcia-Tellado, Eur. J. Org. Chem. 2014, 198–205.
- 18Although the Michael addition step precludes the preparation of 3-substituted and 3,4-disubstituted furans, the stability of 3-furfuryl halides (in stark contrast to their 2-substituted homologues) enables ready access to 3-furfuryl derivatives that can be used in the total syntheses of various 3-substituted furan natural products. For the difference in stability between 3- and 2-furfuryl derivatives, see: E. Sherman, E. D. Amstutz, J. Am. Chem. Soc. 1950, 72, 2195–2199.
- 19Examples of total syntheses of 3-substituted furan natural products using 3-furfuryl derivatives:
- 19aS. P. Tanis, Tetrahedron Lett. 1982, 23, 3115–3118;
- 19bY. Noda, S. Ugajin, A. Yamanaka, N. Mamiya, Heterocycles 2011, 83, 2265–2269;
- 19cD. X. Tan, Z. J. Xu, H. J. Chen, Y. Wu, J. You, Eur. J. Org. Chem. 2016, 946–957;
- 19dS. Serra, Mar. Drugs 2019, 17, 245.
- 20The ready availability of various 3,4-disubstituted furan precursors also facilitates total syntheses of 3,4-disubstituted furan natural products. For examples, see:
- 20aS. P. Tanis, D. B. Head, Tetrahedron Lett. 1982, 23, 5509–5512;
- 20bZ. Z. Song, M. S. Ho, H. N. C. Wong, J. Org. Chem. 1994, 59, 3917–3926.
- 21For the role of water in the generation of active Pd0 complexes from PdII in the Heck reaction, see:
- 21aF. Ozawa, A. Kubo, T. Hayashi, Chem. Lett. 1992, 21, 2177–2180;
- 21bC. Amatore, A. Jutand, A. Thuilliez, Organometallics 2001, 20, 3241–3249.
- 22
- 22aT. J. Jeffery, J. Chem. Soc. Chem. Commun. 1984, 1287–1289;
- 22bT. Jeffery, Tetrahedron Lett. 1985, 26, 2667–2670.
- 23E. Bures, P. G. Spinazze, G. Beese, I. R. Hunt, C. Rogers, B. A. Keay, J. Org. Chem. 1997, 62, 8741–8749.
- 24
- 24aM. R. Netherton, G. C. Fu, Org. Lett. 2001, 3, 4295–4298;
- 24bA. F. Littke, G. C. Fu, J. Am. Chem. Soc. 2001, 123, 6989–7000.
- 25Examples of 2-trifluoromethylfurans: J. Wang, S. Chen, W. Wu, S. Wen, Z. Weng, J. Org. Chem. 2019, 84, 15685–15696.
- 26
- 26aP. Kirsch, Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications, Wiley-VCH, Weinheim, 2013, pp. 299–350;
10.1002/9783527651351.ch9 Google Scholar
- 26bM. N. Gomes, E. N. Muratov, M. Pereira, J. C. Peixoto, L. P. Rosseto, P. V. L. Cravo, C. H. Andrade, B. J. Neves, Molecules 2017, 22, 1210–1235.
- 27S. J. Hayes, D. W. Knight, A. W. T. Smith, M. J. O'Halloran, Tetrahedron Lett. 2010, 51, 717–719.
- 28D. Kalaitzakis, M. Triantafyllakis, I. Alexopoulou, M. Sofiadis, G. Vassilikogiannakis, Angew. Chem. Int. Ed. 2014, 53, 13201–13205; Angew. Chem. 2014, 126, 13417–13421.
- 29
- 29aRef. [8a];
- 29bRef [8d];
- 29cRef [8e].
- 30Ref [8c].
- 31
- 31aAlthough plakorsin D methyl ester has not been prepared de novo, it, along with another furanylidene P. simplex natural product, has been demonstrated to arise from the FeCl3-mediated rearrangement of the cyclic endoperoxide analogue of plakodiepoxide derived from P. simplex sponges; see Ref. [7b].
- 31bR. J. Lee, M. R. Lindley, G. J. Pritchard, M. C. Kimber, Chem. Commun. 2017, 53, 6327–6330.
- 32H. Ishibashi, T. Tabata, T. Kobayashi, I. Takamuro, M. Ikeda, Chem. Pharm. Bull. 1991, 39, 2878–2882.
- 33For the relative stabilities of 2-furfuryl halides, see:
- 33aJ. E. Zanetti, J. Am. Chem. Soc. 1927, 49, 1061–1065;
- 33bJ. E. Zanetti, J. T. Bashour, J. Am. Chem. Soc. 1939, 61, 2249–2251.
- 34Performing the reaction at the melting point of PPh3 allows it to be used as a solvent: “Triphenylphosphine”: J. E. Cobb, C. M. Cribbs, B. R. Henke, D. E. Uehling, e-EROS Encyclopedia of Reagents for Organic Synthesis, Wiley, Hoboken, 2005; https://doi.org/10.1002/047084289X.rt366.
- 35R. M. Boden, Synthesis 1975, 784.
- 36H. Tanaka, Y. Takaya, J. Toyoda, T. Yasuda, M. Sato, J. Murata, H. Murata, K. Kaburagi, O. Iida, K. Sugimura, E. Sakai, Phytochem. Lett. 2015, 11, 32–36.
- 37
- 37aT. Kato, M. Tanemura, S. Kanno, T. Suzuki, Y. Kitahara, Bioorg. Chem. 1971, 1, 84–90;
- 37bW. Kramp, F. Bohlmann, Liebigs Ann. Chem. 1986, 226–233.
- 38A similar E2-elimination has been observed in the basic decomposition of β-phenethylphosphonium bromides: S. Alunni, G. Giulietti, Z. Naturforsch. B 2014, 38, 115–116.
10.1515/znb-1983-0122 Google Scholar
- 39An example of an E-selective Wittig reaction: J. S. Oh, B. H. Kim, Y. G. Kim, Tetrahedron Lett. 2004, 45, 3925–3928.
- 40R. Robiette, J. Richardson, V. K. Aggarwal, J. N. Harvey, J. Am. Chem. Soc. 2006, 128, 2394–2409.
- 41Examples include alkylations of (3-furylmethyl)indate[41] and 2,2′-di-3-methylfurylmercury with prenyl,[42a] geranyl,[42b,c] and farnesyl[42d] bromides; AuI-,[43a] IrI-,[43b] and PdII[43c,d]-catalyzed cycloisomerizations of (Z)-2-en-4-yn-1-ols; reductions of prenyl-[44a] and geranyl[44b-d]-substituted furanones; and cyclizations of α-allenic alcohols.[45] In the case of mikanifuran, alkylation of geranyl p-tolyl sulfone with the allylic chloride derivative[46] prepared from rosefuran has also been reported. See the Supporting Information for a full list of previous syntheses.
- 42
- 42aL. K. Jie, S. F. Marcel, Methods Enzymol. 1981, 72, 443–471;
- 42bY. Groweiss, A. Kashman, Cell. Mol. Life Sci. 1978, 34, 299;
- 42cA. B. Imbs, Russ. J. Mar. Biol. 2013, 39, 153–168.
- 43T. Kimura, A. Tajima, Y. Inahashi, M. Iwatsuki, H. Kasai, T. Mokudai, Y. Niwano, K. Shiomi, Y. Takahashi, S. Omura, T. Nakashima, J. Gen. Appl. Microbiol. 2018, 64, 62–67.
- 44In addition to the strategies used for the preparation of plakorsin natural products and 2,3-disubstituted furanoterpenes, rearrangements of cyclic endoperoxides (Ref. [31b]) and catalytic isomerization of cyclic epoxyalkynols have also been reported for the synthesis of 11 M5 and its methyl ester. Other syntheses include lithiation/alkylation of 3-iodofurans; Sonogashira and Negishi couplings of methyl 4,5-dibromofurano-2-carboxylate; electrophilic aromatic substitution of methyl 3-methyl-2-furoate; and reduction/iodination/reduction of 3-ethoxycarbonylfurans. See the Supporting Information for a list of previous syntheses.
- 45The coupling constants (J) for the vinyl protons of methyl (Z)-β-alkoxyacrylates range from 6.7 to 7.2 Hz, in contrast to values of approximately 12 Hz for corresponding E isomers. For examples, see:
- 45aS. P. Singh, J. S. O'Donnell, A. L. Schwan, Org. Biomol. Chem. 2010, 8, 1712–1717;
- 45bY. Sarrafi, M. Sadatshahabi, K. Alimohammadi, M. Tajbakhsh, Green Chem. 2011, 13, 2851–2858;
- 45cD. E. Bergbreiter, Y-.C. Yang, C. E. Hobbs, J. Org. Chem. 2011, 76, 6912–6917.
- 46Examples of PMe3-catalyzed addition of tertiary alcohols onto methyl propiolate:
- 46aK. Sato, M. Sasaki, Org. Lett. 2005, 7, 2441–2444;
- 46bK. Sato, M. Sasaki, Tetrahedron 2007, 63, 5977–6003;
- 46cJ. A. Davy, J. W. Mason, B. Moreau, J. E. Wulff, J. Org. Chem. 2012, 77, 6332–6339;
- 46dM. Kunitake, T. Oshima, K. Konoki, M. Ebine, K. Torikai, M. Murata, T. Oishi, J. Org. Chem. 2014, 79, 4948–4962.
- 47While an alkynyl group would lower the pKa of a tertiary alcohol by approximately 2 units, replacing one of the methyl groups in tert-butanol by a CF3 group would lower the pKa from 17 to 12.6 (a change of 4.4 units). See Ref. [17c].
- 48DABCO is known to quantitatively dimerize methyl propiolate to (E)-hex-2-en-4-ynedioic acid dimethyl ester:
- 48aY. Matsuya, K. Hayashi, H. Nemoto, J. Am. Chem. Soc. 2003, 125, 646–647;
- 48bL.-H. Zhou, X.-Q. Yu, L. Pu, Tetrahedron Lett. 2010, 51, 425–427;
- 48cF. Pünner, G. Hilt, Chem. Commun. 2012, 48, 3617–3619;
- 48dJ.-H. Choi, C.-M. Park, Adv. Synth. Catal. 2018, 360, 3553–3562.