Polymorphism of 2D Imine Covalent Organic Frameworks
Yusen Li
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
These authors contributed equally to this work.
Search for more papers by this authorLinshuo Guo
School of Physical Science and Technology, Shanghai Tech University, Shanghai, 201210 China
These authors contributed equally to this work.
Search for more papers by this authorYongkang Lv
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorZiqiang Zhao
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorProf. Yanhang Ma
School of Physical Science and Technology, Shanghai Tech University, Shanghai, 201210 China
Search for more papers by this authorProf. Weihua Chen
College of Chemistry and Green Catalysis Center, Zhengzhou University, Henan, 450001 China
Search for more papers by this authorGuolong Xing
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorProf. Donglin Jiang
Department of Chemistry, Faculty of Science, National University of Singapore, 3 Science Drive, Singapore, 117543 Singapore
Search for more papers by this authorCorresponding Author
Prof. Long Chen
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorYusen Li
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
These authors contributed equally to this work.
Search for more papers by this authorLinshuo Guo
School of Physical Science and Technology, Shanghai Tech University, Shanghai, 201210 China
These authors contributed equally to this work.
Search for more papers by this authorYongkang Lv
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorZiqiang Zhao
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorProf. Yanhang Ma
School of Physical Science and Technology, Shanghai Tech University, Shanghai, 201210 China
Search for more papers by this authorProf. Weihua Chen
College of Chemistry and Green Catalysis Center, Zhengzhou University, Henan, 450001 China
Search for more papers by this authorGuolong Xing
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorProf. Donglin Jiang
Department of Chemistry, Faculty of Science, National University of Singapore, 3 Science Drive, Singapore, 117543 Singapore
Search for more papers by this authorCorresponding Author
Prof. Long Chen
Department of Chemistry, Institute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorGraphical Abstract
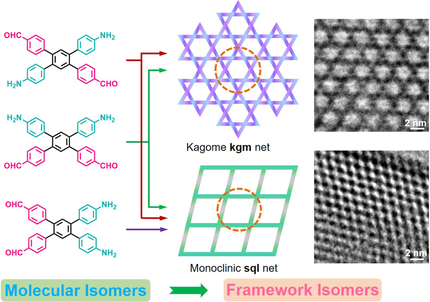
A strategy was developed for selective growth of isomeric covalent organic frameworks by designing monomer isomers and tuning reaction conditions. Three A2B2 type tetraphenyl benzene monomers (p-, m-, and o-TetPB) afford five different 2D TetPB-COF isomers that exhibit selective adsorption of vitamin B12 owing to a great difference in their pore shape and size.
Abstract
We designed and synthesized A2B2 type tetraphenyl benzene monomers (p-, m-, and o-TetPB) which have the para-, meta, and ortho-substituted isomeric structures, for the direct construction of isomeric frameworks. Interestingly, both kagome (kgm) and monoclinic square (sql) framework isomers are produced from either p-TetPB (C2h symmetry) or m-TetPB (C2v symmetry) by changing reaction solvents, while their isomeric structures are characterized by X-ray diffraction, computational simulation, microscopy, and sorption isotherm measurements. Only sql frameworks was formed for o-TetPB (C2v symmetry), irrespective of reaction solvents. These results disclose a unique feature in the framework structural formation, that is, the geometry of monomers directs and dominates the lattice growth process while the solvent plays a role in the perturbation of chain growth pattern. The isomeric frameworks exhibit highly selective adsorption of vitamin B12 owing to pore shape and size differences.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202015130-sup-0001-misc_information.pdf11.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Datta, M. L. Saha, P. J. Stang, Acc. Chem. Res. 2018, 51, 2047–2063;
- 1bO. N. Kavanagh, D. M. Croker, G. M. Walker, M. J. Zaworotko, Drug Discovery Today 2019, 24, 796–804.
- 2
- 2aZ.-J. Guan, F. Hu, J.-J. Li, Z.-R. Wen, Y.-M. Lin, Q.-M. Wang, J. Am. Chem. Soc. 2020, 142, 2995–3001;
- 2bS. Tian, Y. Z. Li, M. B. Li, J. Yuan, J. Yang, Z. Wu, R. Jin, Nat. Commun. 2015, 6, 8667.
- 3K. Jie, Y. Zhou, E. Li, F. Huang, Acc. Chem. Res. 2018, 51, 2064–2072.
- 4T. D. Bennett, A. K. Cheetham, Acc. Chem. Res. 2014, 47, 1555–1562.
- 5J. Ma, L. D. Tran, A. J. Matzger, Cryst. Growth Des. 2016, 16, 4148–4153.
- 6J. Pang, S. Yuan, J. Qin, C. Liu, C. Lollar, M. Wu, D. Yuan, H.-C. Zhou, M. Hong, J. Am. Chem. Soc. 2017, 139, 16939–16945.
- 7F. Igoa, S. Martínez, K. P. S. Zanoni, J. Castiglioni, L. Suescun, J. González-Platas, A. S. S. D. Camargo, C. Kremera, J. Torres, CrystEngComm 2018, 20, 4942–4953.
- 8
- 8aB. Karadeniz, D. Žilić, I. Huskić, L. S. Germann, A. M. Fidelli, S. Muratović, I. Lončarić, M. Etter, R. E. Dinnebier, D. Barišić, N. Cindro, T. Islamoglu, O. K. Farha, T. Friščić, K. Užarević, J. Am. Chem. Soc. 2019, 141, 19214–19220;
- 8bS.-H. Lo, L. Feng, K. Tan, Z. Huang, S. Yuan, K.-Y. Wang, B.-H. Li, W.-L. Day, G. S. Liu, S. Yang, C.-C. Tao, T.-T. Luo, C.-H. Lin, S.-L. Wang, S. J. L. Billinge, K.-L. Lu, Y. J. Chabal, X. Zou, H.-C. Zhou, Nat. Chem. 2020, 12, 90–97.
- 9
- 9aS. Y. Ding, W. Wang, Chem. Soc. Rev. 2013, 42, 548–568;
- 9bY. Jin, Y. Hu, W. Zhang, Nat. Rev. Chem. 2017, 1, 0056;
- 9cZ. Xie, Y. Li, L. Chen, D. Jiang, Acta Polym. Sin. 2016, 12, 1621–1634;
- 9dQ. Yang, D. Liu, C. Zhong, J.-R. Li, Chem. Rev. 2013, 113, 8261–8323;
- 9eJ. Á. Martín-Illán, D. Rodríguez-San-Miguel, C. Franco, I. Imaz, D. Maspoch, J. Puigmartí-Luis, F. Zamora, Chem. Commun. 2020, 56, 6704–6707.
- 10
- 10aK. Geng, T. He, R. Liu, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang, D. Jiang, Chem. Rev. 2020, 120, 8814–8933;
- 10bN. Huang, L. Zhai, D. E. Coupry, M. A. Addicoat, K. Okushita, K. Nishimura, T. Heine, D. Jiang, Nat. Commun. 2016, 7, 12325.
- 11
- 11aP. F. Wei, M. Z. Qi, Z. P. Wang, S. Y. Ding, W. Yu, Q. Liu, L. K. Wang, H. Z. Wang, W. K. An, W. Wang, J. Am. Chem. Soc. 2018, 140, 4623–4631;
- 11bX. Wang, L. Chen, S. Y. Chong, M. A. Little, Y. Wu, W. H. Zhu, R. Clowes, Y. Yan, M. A. Zwijnenburg, R. S. Sprick, A. I. Cooper, Nat. Chem. 2018, 10, 1180–1189.
- 12
- 12aD. Bessinger, L. Ascherl, F. Auras, T. Bein, J. Am. Chem. Soc. 2017, 139, 12035–12042;
- 12bH. Ding, J. Li, G. Xie, G. Lin, R. Chen, Z. Peng, C. Yang, B. Wang, J. Sun, C. Wang, Nat. Commun. 2018, 9, 5234.
- 13
- 13aQ. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu, Y. Yan, J. Am. Chem. Soc. 2015, 137, 8352–8355;
- 13bL. Bai, S. Z. F. Phua, W. Q. Lim, A. Jana, Z. Luo, H. P. Tham, L. Zhao, Q. Gao, Y. Zhao, Chem. Commun. 2016, 52, 4128–4131;
- 13cG. Zhang, X. Li, Q. Liao, Y. Liu, K. Xi, W. Huang, X. Jia, Nat. Commun. 2018, 9, 2785.
- 14
- 14aS. Wang, Q. Wang, P. Shao, Y. Han, X. Gao, L. Ma, S. Yuan, X. Ma, J. Zhou, X. Feng, B. Wang, J. Am. Chem. Soc. 2017, 139, 4258–4261;
- 14bX. Chen, Y. Li, L. Wang, Y. Xu, A. Nie, Q. Li, F. Wu, W. Sun, X. Zhang, R. Vajtai, P. M. Ajayan, L. Chen, Y. Wang, Adv. Mater. 2019, 31, 1901640.
- 15
- 15aX. Liu, D. Huang, C. Lai, G. Zeng, L. Qin, H. Wang, H. Yi, B. Li, S. Liu, M. Zhang, R. Deng, Y. Fu, L. Li, W. Xue, S. Chen, Chem. Soc. Rev. 2019, 48, 5266–5302;
- 15bZ. Li, N. Huang, K. H. Lee, Y. Feng, S. Tao, Q. Jiang, Y. Nagao, S. Irle, D. Jiang, J. Am. Chem. Soc. 2018, 140, 12374–12377;
- 15cH. Yuan, N. Li, J. Linghu, J. Dong, Y. Wang, A. Karmakar, J. Yuan, M. Li, P. J. S. Buenconsejo, G. Liu, H. Cai, S. J. Pennycook, N. Singh, D. Zhao, ACS Sens. 2020, 5, 1474–1481.
- 16
- 16aT. Ma, J. Li, J. Niu, L. Zhang, A. S. Etman, C. Lin, D. Shi, P. Chen, L.-H. Li, X. Du, J. Sun, W. Wang, J. Am. Chem. Soc. 2018, 140, 6763–6766;
- 16bR.-R. Liang, F.-Z. Cui, R.-H. A, Q.-Y. Qi, X. Zhao, CCS Chem. 2020, 2, 139–145;
- 16cY.-P. Mo, X.-H. Liu, D. Wang, ACS Nano 2017, 11, 11694–11700.
- 17F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe, O. M. Yaghi, J. Am. Chem. Soc. 2009, 131, 4570–4571.
- 18
- 18aT.-Y. Zhou, S.-Q. Xu, Q. Wen, Z.-F. Pang, X. Zhao, J. Am. Chem. Soc. 2014, 136, 15885–15888;
- 18bZ.-F. Pang, T.-Y. Zhou, R.-R. Liang, Q.-Y. Qi, X. Zhao, Chem. Sci. 2017, 8, 3866–3870;
- 18cF. Auras, L. Ascherl, A. H. Hakimioun, J. T. Margraf, F. C. Hanusch, S. Reuter, D. Bessinger, M. Doblinger, C. Hettstedt, K. Karaghiosoff, J. Am. Chem. Soc. 2016, 138, 16703–16710.
- 19
- 19aA. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger, O. M. Yaghi, Science 2005, 310, 1166–1170;
- 19bP. Kuhn, M. Antonietti, A. Thomas, Angew. Chem. Int. Ed. 2008, 47, 3450–3453; Angew. Chem. 2008, 120, 3499–3502.
- 20
- 20aY. Li, W. Chen, G. Xing, D. Jiang, L. Chen, Chem. Soc. Rev. 2020, 49, 2852–2868;
- 20bY. Li, Q. Chen, T. Xu, Z. Xie, J. Liu, X. Yu, S. Ma, T. Qin, L. Chen, J. Am. Chem. Soc. 2019, 141, 13822–13828;
- 20cW. Hao, D. Chen, Y. Li, Z. Yang, G. Xing, J. Li, L. Chen, Chem. Mater. 2019, 31, 8100–8105;
- 20dX. Yan, H. Liu, Y. Li, W. Chen, T. Zhang, Z. Zhao, G. Xing, L. Chen, Macromolecules 2019, 52, 7977–7983;
- 20eD. Chen, W. Chen, G. Xing, T. Zhang, L. Chen, Chem. Eur. J. 2020, 26, 8377–8381;
- 20fB. Zhang, X. Song, Y. Li, Y. Li, Z. Peng, L. Ye, L. Chen, Chem. Commun. 2020, 56, 3253–3256.
- 21G. Das, T. Skorjanc, S. K. Sharma, F. Gándara, M. Lusi, D. S. S. Rao, S. Vimala, S. K. Prasad, J. Raya, D. S. Han, R. Jagannathan, J.-C. Olsen, A. Trabolsi, J. Am. Chem. Soc. 2017, 139, 9558–9565.
- 22D. C. Hodgkin, J. Kamper, M. Mackay, J. Pickworth, K. N. Trueblood, J. G. White, Nature 1956, 178, 64–66.