Potent Trivalent Inhibitors of Thrombin through Hybridization of Salivary Sulfopeptides from Hematophagous Arthropods
In memory of Chris Abell
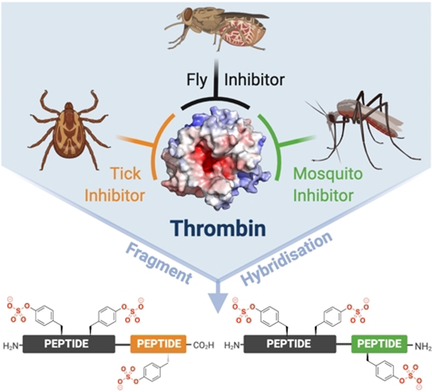
Graphical Abstract
The rational design of a novel class of trivalent thrombin inhibitors is described through the hybridization of natural sulfopeptides produced by blood feeding organisms. The hybrid sulfopeptides were rapidly assembled using peptide ligation chemistry and shown to exhibit femtomolar inhibition constants against thrombin. A lead inhibitor also showed potent inhibition of thrombin generation and platelet aggregation in vitro, as well as antithrombotic effects in a murine model.
Abstract
Blood feeding arthropods, such as leeches, ticks, flies and mosquitoes, provide a privileged source of peptidic anticoagulant molecules. These primarily operate through inhibition of the central coagulation protease thrombin by binding to the active site and either exosite I or exosite II. Herein, we describe the rational design of a novel class of trivalent thrombin inhibitors that simultaneously block both exosites as well as the active site. These engineered hybrids were synthesized using tandem diselenide-selenoester ligation (DSL) and native chemical ligation (NCL) reactions in one-pot. The most potent trivalent inhibitors possessed femtomolar inhibition constants against α-thrombin and were selective over related coagulation proteases. A lead hybrid inhibitor possessed potent anticoagulant activity, blockade of both thrombin generation and platelet aggregation in vitro and efficacy in a murine thrombosis model at 1 mg kg−1. The rational engineering approach described here lays the foundation for the development of potent and selective inhibitors for a range of other enzymatic targets that possess multiple sites for the disruption of protein–protein interactions, in addition to an active site.
Conflict of interest
The authors declare no conflict of interest.