Palladium-Catalyzed Site-Selective [3+2] Annulation via Benzylic and meta C−H Bond Activation
Graphical Abstract
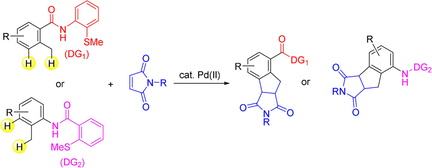
Abstract
The palladium-catalyzed [3+2] annulation of aromatic amides with maleimides via the activation of ortho benzylic C−H and meta C−H bonds is reported. Carboxamide and anilide type substrates that contain a 2-methylthiophenyl group both participate in this [3+2] annulation, indicating that the presence of a 2-methylthiophenyl directing group is a key for the success of the reaction. The first C−H bond activation at the benzylic C−H bond is followed by a second C−H bond activation at the meta C−H bond to give five-membered cyclic products. The cleavage of these C−H bonds is irreversible.