Role of Pyridinic Nitrogen in the Mechanism of the Oxygen Reduction Reaction on Carbon Electrocatalysts
Graphical Abstract
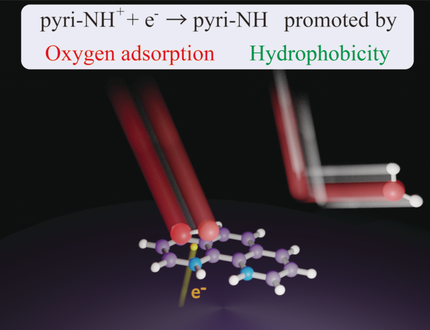
In N-doped carbon catalysts, pyridinic nitrogen (pyri-N) provides active sites for the oxygen reduction reaction (ORR). The roles of pyri-N in the ORR mechanism were investigated using pyri-N-containing molecules as model catalysts. In acidic media, pyri-N species were protonated to pyri-NH+ and then reduced upon the simultaneous thermal adsorption of O2. The reduction was accelerated by hydrophobic environments.
Abstract
The introduction of pyridinic nitrogen (pyri-N) into carbon-based electrocatalysts for the oxygen reduction reaction is considered to create new active sites. Herein, the role of pyri-N in such catalysts was investigated from a mechanistic viewpoint using carbon black (CB)-supported pyri-N-containing molecules as model catalysts; the highest activity was observed for 1,10-phenanthroline/CB. X-ray photoemission spectroscopy showed that in acidic electrolytes, both pyri-N atoms of 1,10-phenanthroline could be protonated to form pyridinium ions (pyri-NH+). In O2-saturated electrolytes, one of the pyri-NH+ species was reduced to pyri-NH upon the application of a potential; no such reduction was observed in N2-saturated electrolytes. This behavior was ascribed to electrochemical reduction of pyri-NH+ occurring simultaneously with the thermal adsorption of O2, as supported by DFT calculations. According to these calculations, the coupled reduction was promoted by hydrophobic environments.