Free-Radical Membrane Protein Footprinting by Photolysis of Perfluoroisopropyl Iodide Partitioned to Detergent Micelle by Sonication
Graphical Abstract
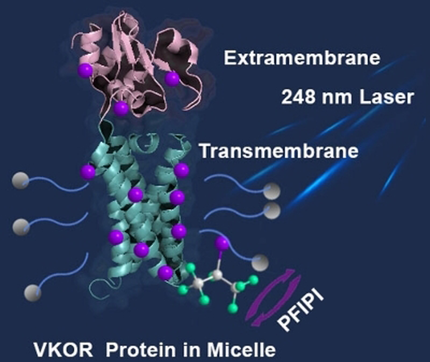
Laser-mediated free-radical footprinting of an integral membrane protein (IMP) is described. Compared to canonical footprinting, the approach exploits tip sonication to ensure efficient transport of a highly hydrophobic perfluoroalkyl iodide to footprint transmembrane domains. This approach, which is amenable to native IMP structures, yields 100 % coverage for tyrosine, and it is compatible with standard proteomic mass spectrometric analysis.
Abstract
A free-radical footprinting approach is described for integral membrane protein (IMP) that extends, significantly, the “fast photochemical oxidation of proteins” (FPOP) platform. This new approach exploits highly hydrophobic perfluoroisopropyl iodide (PFIPI) together with tip sonication to ensure efficient transport into the micelle interior, allowing laser dissociation and footprinting of the transmembrane domains. In contrast to water soluble footprinters, PFIPI footprints both the hydrophobic intramembrane and the hydrophilic extramembrane domains of the IMP vitamin K epoxide reductase (VKOR). The footprinting is fast, giving high coverage for Tyr (100 %) and Trp. The incorporation of the reagent with sonication does not significantly affect VKOR's enzymatic function, and tyrosine iodination does not compromise protease digestion and the subsequent analysis. The locations for the modifications are largely consistent with the corresponding solvent accessibilities, recommending this approach for future membrane protein footprinting.
Conflict of interest
The authors declare no conflict of interest.