Photoresponsive Prion-Mimic Foldamer to Induce Controlled Protein Aggregation
Graphical Abstract
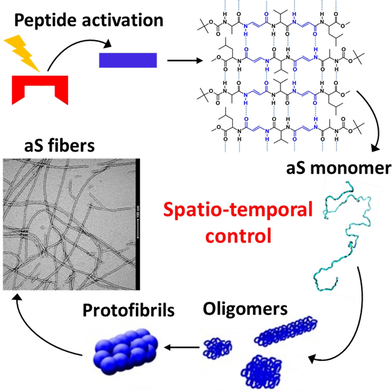
A photo-responsive prion-like foldamer is designed to act as spatio-temporal molecular template, which can be activated by selective photo-irradiation without changing other experimental conditions. It is able to induce a fast transition to the β-sheet conformation for aS monomers that undergo fibrillar polymerization. This method allows the study of protein aggregation relevant to misfolding diseases.
Abstract
Proteins reconfigure their 3D-structure, and consequently their function, under the control of specific molecular interactions that sense, process and transmit information from the surrounding environment. When this fundamental process is hampered, many pathologies occur as in the case of protein misfolding diseases. In this work, we follow the early steps of α-synuclein (aS) aggregation, a process associated with Parkinson's disease etiopathogenesis, that is promptly promoted by a light-mediated binding between the protein and a photoactive foldamer. The latter can switch between two conformations, one of which generates supramolecular fibrillar seeds that act as molecular templates able to induce a fast β-sheet transition for aS monomers that successively undergo fibrillar polymerization. The proposed method represents a powerful tool to study protein aggregation relevant to misfolding diseases in a controlled and inducible system.