Carbon Monoxide Activation by a Molecular Aluminium Imide: C−O Bond Cleavage and C−C Bond Formation
Andreas Heilmann
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorDr. Jamie Hicks
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorDr. Petra Vasko
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland
Search for more papers by this authorCorresponding Author
Prof. Jose M. Goicoechea
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorCorresponding Author
Prof. Simon Aldridge
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorAndreas Heilmann
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorDr. Jamie Hicks
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorDr. Petra Vasko
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland
Search for more papers by this authorCorresponding Author
Prof. Jose M. Goicoechea
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorCorresponding Author
Prof. Simon Aldridge
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorGraphical Abstract
Abstract
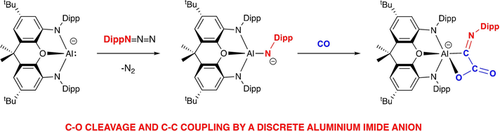
Anionic molecular imide complexes of aluminium are accessible via a rational synthetic approach involving the reactions of organo azides with a potassium aluminyl reagent. In the case of K2[(NON)Al(NDipp)]2 (NON=4,5-bis(2,6-diisopropylanilido)-2,7-di-tert-butyl-9,9-dimethyl-xanthene; Dipp=2,6-diisopropylphenyl) structural characterization by X-ray crystallography reveals a short Al−N distance, which is thought primarily to be due to the low coordinate nature of the nitrogen centre. The Al−N unit is highly polar, and capable of the activation of relatively inert chemical bonds, such as those found in dihydrogen and carbon monoxide. In the case of CO, uptake of two molecules of the substrate leads to C−C coupling and C≡O bond cleavage. Thermodynamically, this is driven, at least in part, by Al−O bond formation. Mechanistically, a combination of quantum chemical and experimental observations suggests that the reaction proceeds via exchange of the NR and O substituents through intermediates featuring an aluminium-bound isocyanate fragment.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201916073-sup-0001-misc_information.pdf1.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Advances in Fischer–Tropsch Synthesis Catalysts and Catalysis (Eds.: ), CRC, Boca Raton, 2009;
- 1bA. Y. Khodakov, W. Chu, P. Fongarland, Chem. Rev. 2007, 107, 1692–1744;
- 1cC. K. Rofer-DePoorter, Chem. Rev. 1981, 81, 447–474;
- 1dC. Masters, Adv. Organomet. Chem. 1979, 17, 61–103.
- 2s-Block:
- 2aL. Gmelin, Ann. Phys. Chem. 1825, 4, 31–62;
10.1002/andp.18250800504 Google Scholar
- 2bW. Büchner, Helv. Chim. Acta 1963, 46, 2111–2120;
- 2cS. Coluccia, E. Garrone, E. Guglielminotti, A. Zecchina, J. Chem. Soc. Faraday Trans. 1 1981, 77, 1063–1073;
- 2dP. W. Lednor, P. C. Versloot, J. Chem. Soc. Chem. Commun. 1983, 284–285;
- 2eR. Lalrempuia, C. E. Kefalidis, S. J. Bonyhady, B. Schwarze, L. Maron, A. Stasch, C. Jones, J. Am. Chem. Soc. 2015, 137, 8944–8947;
- 2fM. D. Anker, M. S. Hill, J. P. Lowe, M. F. Mahon, Angew. Chem. Int. Ed. 2015, 54, 10009–10011; Angew. Chem. 2015, 127, 10147–10149.
- 3d-Block:
- 3aP. A. Bianconi, I. D. Williams, M. P. Engeler, S. J. Lippard, J. Am. Chem. Soc. 1986, 108, 311–313;
- 3bP. A. Bianconi, R. N. Vrtis, C. P. Rao, I. D. Williams, M. P. Engeler, S. J. Lippard, Organometallics 1987, 6, 1968–1977;
- 3cR. N. Vrtis, C. P. Rao, S. G. Bott, S. J. Lippard, J. Am. Chem. Soc. 1988, 110, 7564–7566;
- 3dJ. D. Protasiewicz, S. J. Lippard, J. Am. Chem. Soc. 1991, 113, 6564–6570;
- 3eE. M. Carnahan, J. D. Protasiewicz, S. J. Lippard, Acc. Chem. Res. 1993, 26, 90–97;
- 3fA. J. M. Miller, J. A. Labinger, J. E. Bercaw, J. Am. Chem. Soc. 2008, 130, 11874–11875;
- 3gT. Watanabe, Y. Ishida, T. Matsuo, H. Kawaguchi, J. Am. Chem. Soc. 2009, 131, 3474–3475.
- 4f-Block:
- 4aW. J. Evans, J. W. Grate, L. A. Hughes, H. Zhang, J. L. Atwood, J. Am. Chem. Soc. 1985, 107, 3728–3730;
- 4bO. T. Summerscales, F. G. N. Cloke, P. B. Hitchcock, J. C. Green, N. Hazari, Science 2006, 311, 829–831;
- 4cO. T. Summerscales, F. G. N. Cloke, P. B. Hitchcock, J. C. Green, N. Hazari, J. Am. Chem. Soc. 2006, 128, 9602–9603;
- 4dW. J. Evans, D. S. Lee, J. W. Ziller, N. Kaltsoyannis, J. Am. Chem. Soc. 2006, 128, 14176–14184;
- 4eA. S. Frey, F. G. N. Cloke, P. B. Hitchcock, I. J. Day, J. C. Green, G. Aitken, J. Am. Chem. Soc. 2008, 130, 13816–13817;
- 4fP. L. Arnold, Z. R. Turner, R. M. Bellabarba, R. P. Tooze, Chem. Sci. 2011, 2, 77–79;
- 4gS. M. Mansell, N. Kaltsoyannis, P. L. Arnold, J. Am. Chem. Soc. 2011, 133, 9036–9051;
- 4hB. M. Gardner, J. C. Stewart, A. L. Davis, J. McMaster, W. Lewis, A. J. Blake, S. T. Liddle, Proc. Natl. Acad. Sci. USA 2012, 109, 9265–9270;
- 4iB. Wang, G. Luo, M. Nishiura, Y. Luo, Z. Hou, J. Am. Chem. Soc. 2017, 139, 16967–16973.
- 5p-Block:
- 5aH. Braunschweig, T. Dellermann, R. D. Dewhurst, W. C. Ewing, K. Hammond, J. O. C. Jimenez-Halla, T. Kramer, I. Krummenacher, J. Mies, A. K. Phukan, A. Vargas, Nat. Chem. 2013, 5, 1025–1028, See also ref. 4i and
- 5bX. P. Wang, Z. L. Zhu, Y. Peng, H. Lei, J. C. Fettinger, P. P. Power, J. Am. Chem. Soc. 2009, 131, 6912–6913;
- 5cZ. D. Brown, P. P. Power, Inorg. Chem. 2013, 52, 6248–6259;
- 5dR. Y. Kong, M. R. Crimmin, J. Am. Chem. Soc. 2018, 140, 13614–13617 (mixed p/d-block);
- 5eA. V. Protchenko, P. Vasko, D. C. H. Do, J. Hicks, M. A. Fuentes, C. Jones, S. Aldridge, Angew. Chem. Int. Ed. 2019, 58, 1808–1812; Angew. Chem. 2019, 131, 1822–1826.
- 6Two-centre reduction of CO by boron/phosphorus frustrated Lewis pairs has been reported:
- 6aM. Sajid, L.-M. Elmer, C. Rosorius, C. G. Daniliuc, S. Grimme, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2013, 52, 2243–2246; Angew. Chem. 2013, 125, 2299–2302;
- 6bR. Dobrovetsky, D. W. Stephan, J. Am. Chem. Soc. 2013, 135, 4974–4977;
- 6cM. Devillard, B. de Bruin, M. I. Siegler, J. I. van der Vlugt, Chem. Eur. J. 2017, 23, 13628; See also:
- 6dM. Arrowsmith, J. Böhnke, H. Braunschweig, M. A. Celik, Angew. Chem. Int. Ed. 2017, 56, 14287–14292; Angew. Chem. 2017, 129, 14475–14480;
- 6eH. Wang, L. Wu, Z. Lin, Z. Xie, Angew. Chem. Int. Ed. 2018, 57, 8708–8713; Angew. Chem. 2018, 130, 8844–8849.
- 7
- 7aM. Majumdar, I. Omlor, C. B. Yildiz, A. Azizoglu, V. Huch, D. Scheschekewitz, Angew. Chem. Int. Ed. 2015, 54, 8746–8750; Angew. Chem. 2015, 127, 8870–8874;
- 7bY. Wang, A. Kostenko, T. J. Hadlington, M.-P. Luecke, S. Yao, M. Driess, J. Am. Chem. Soc. 2019, 141, 626–634;
- 7cY. Xiong, S. Yao, T. Szilvási, A. Ruzicka, M. Driess, Chem. Commun. 2020, 56, 747–750.
- 8
- 8aJ. Hicks, P. Vasko, J. M. Goicoechea, S. Aldridge, Nature 2018, 557, 92–95;
- 8bJ. Hicks, A. Mansikkamäki, P. Vasko, J. M. Goicoechea, S. Aldridge, Nat. Chem. 2019, 11, 237–241;
- 8cJ. Hicks, P. Vasko, J. M. Goicoechea, S. Aldridge, J. Am. Chem. Soc. 2019, 141, 11000–11003;
- 8dJ. Hicks, A. Heilmann, P. Vasko, J. M. Goicoechea, S. Aldridge, Angew. Chem. Int. Ed. 2019, 58, 17265–17268; Angew. Chem. 2019, 131, 17425–17428.
- 9
- 9aJ. Li, X. Li, W. Huang, H. Hu, J. Zhang, C. Cui, Chem. Eur. J. 2012, 18, 15263–15267. During the final stages of revision of this manuscript, a related aluminium imide synthesis was reported:
- 9bM. D. Anker, R. J. Schwamm, M. P. Coles, Chem. Commun. 2020, https://doi.org/10.1039/C9CC09214E.
- 10D. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400–6441; Angew. Chem. 2015, 127, 6498–6541.
- 11
- 11aN. J. Hardman, C. Cui, H. W. Roesky, W. H. Fink, P. P. Power, Angew. Chem. Int. Ed. 2001, 40, 2172–2174;
10.1002/1521-3773(20010601)40:11<2172::AID-ANIE2172>3.0.CO;2-Y CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 2230–2232; See also:
- 11bC. Cui, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, Angew. Chem. Int. Ed. 2000, 39, 4531–4533;
10.1002/1521-3773(20001215)39:24<4531::AID-ANIE4531>3.0.CO;2-K CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 4705–4707; See also:
- 11cH. Zhu, J. Chai, V. Chandrasekhar, H. W. Roesky, J. Magull, D. Vidovic, H.-G. Schmidt, M. Noltemeyer, P. P. Power, W. A. Merrill, J. Am. Chem. Soc. 2004, 126, 9472–9473;
- 11dH. Zhu, Z. Yang, J. Magull, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, Organometallics 2005, 24, 6420–6425.
- 12M. D. Anker, M. Lein, M. P. Coles, Chem. Sci. 2019, 10, 1212–1218.
- 13M. D. Anker, M. P. Coles, Angew. Chem. Int. Ed. 2019, 58, 18261–18265; Angew. Chem. 2019, 131, 18429–18433.
- 14B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán, S. Alvarez, Dalton Trans. 2008, 2832–2838.
- 15Recent examples of H2 activation by early transition metal imides include:
- 15aX. Han, L. Xiang, C. A. Lamsfus, W. Mao, E. Lu, L. Maron, X. Leng, Y. Chen, Chem. Eur. J. 2017, 23, 14728–14732;
- 15bH. S. la Pierre, J. Arnold, F. D. Toste, Angew. Chem. Int. Ed. 2011, 50, 3900–3903; Angew. Chem. 2011, 123, 3986–3989;
- 15cA. M. Geer, C. Tejel, J. A. López, M. A. Ciriano, Angew. Chem. Int. Ed. 2014, 53, 5614–5618; Angew. Chem. 2014, 126, 5720–5724.
- 16For a review of C−H activation by early transition metal imides, see: P. T. Wolczanski, Organometallics 2018, 37, 505–516.
- 17For a previous report of an aluminium carbonyl complex see:
- 17aA. J. Hinchcliffe, J. S. Ogden, D. D. Oswald, J. Chem. Soc. Chem. Commun. 1972, 338–339; For examples of isolable compounds featuring coordination of the CO molecule to a group 13 element centre, see:
- 17bF. Dahcheh, D. Martin, D. W. Stephan, G. Bertrand, Angew. Chem. Int. Ed. 2014, 53, 13159–13163; Angew. Chem. 2014, 126, 13375–13379;
- 17cH. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas, Q. Ye, Nature 2015, 522, 327–330.
- 18CCDC 1972052, 1972053, 1972054, 1972055, 1972056, and 1972057 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.