A General Strategy to Enhance Donor-Acceptor Molecules Using Solvent-Excluding Substituents
Conner A. Hoelzel
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorHang Hu
Department of Chemistry, University of Washington, Seattle, WA, 98105 USA
Molecular Engineering and Sciences Institute, University of Washington, Seattle, WA, 98105 USA
Search for more papers by this authorCharles H. Wolstenholme
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorBasel A. Karim
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorKyle T. Munson
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorKwan Ho Jung
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorHan Zhang
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorProf. Yu Liu
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Dalian Institute of Chemical Physics, Dalian, 116023 China
Search for more papers by this authorProf. Hemant P. Yennawar
Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorProf. John B. Asbury
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorCorresponding Author
Prof. Xiaosong Li
Department of Chemistry, University of Washington, Seattle, WA, 98105 USA
Search for more papers by this authorCorresponding Author
Prof. Xin Zhang
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorConner A. Hoelzel
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorHang Hu
Department of Chemistry, University of Washington, Seattle, WA, 98105 USA
Molecular Engineering and Sciences Institute, University of Washington, Seattle, WA, 98105 USA
Search for more papers by this authorCharles H. Wolstenholme
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorBasel A. Karim
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorKyle T. Munson
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorKwan Ho Jung
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorHan Zhang
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorProf. Yu Liu
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Dalian Institute of Chemical Physics, Dalian, 116023 China
Search for more papers by this authorProf. Hemant P. Yennawar
Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorProf. John B. Asbury
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorCorresponding Author
Prof. Xiaosong Li
Department of Chemistry, University of Washington, Seattle, WA, 98105 USA
Search for more papers by this authorCorresponding Author
Prof. Xin Zhang
Department of Chemistry, Pennsylvania State University, University Park, PA, 16802 USA
Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, PA, 16802 USA
Search for more papers by this authorGraphical Abstract
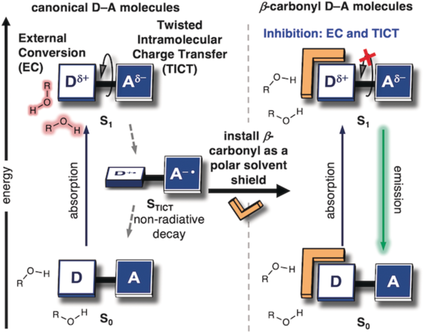
On the bright side: The photophysical behavior of donor-acceptor (D-A) molecules can be improved through installation of a polar, solvent-shielding auxiliary to an amino-donating group. This β-carbonyl substituent generally enhances the fluorescence quantum yield of D-A fluorophores, enabling brighter labeling probes for live-cell imaging.
Abstract
While organic donor-acceptor (D-A) molecules are widely employed in multiple areas, the application of more D-A molecules could be limited because of an inherent polarity sensitivity that inhibits photochemical processes. Presented here is a facile chemical modification to attenuate solvent-dependent mechanisms of excited-state quenching through addition of a β-carbonyl-based polar substituent. The results reveal a mechanism wherein the β-carbonyl substituent creates a structural buffer between the donor and the surrounding solvent. Through computational and experimental analyses, it is demonstrated that the β-carbonyl simultaneously attenuates two distinct solvent-dependent quenching mechanisms. Using the β-carbonyl substituent, improvements in the photophysical properties of commonly used D-A fluorophores and their enhanced performance in biological imaging are shown.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201915744-sup-0001-misc_information.pdf8.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. P. Pelliccioli, J. Wirz, Photochem. Photobiol. Sci. 2002, 1, 441–458;
- 1bY. Chen, M. G. Steinmetz, Org. Lett. 2005, 7, 3729–3732;
- 1cY. Chen, M. G. Steinmetz, J. Org. Chem. 2006, 71, 6053–6060;
- 1dR. S. Givens, M. Rubina, J. Wirz, Photochem. Photobiol. Sci. 2012, 11, 472–488;
- 1eP. Klán, T. Šolomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov, J. Wirz, Chem. Rev. 2013, 113, 119–191;
- 1fA. T. Buck, C. L. Beck, A. H. Winter, J. Am. Chem. Soc. 2014, 136, 8933–8940;
- 1gP. P. Goswami, A. Syed, C. L. Beck, T. R. Albright, K. M. Mahoney, R. Unash, E. A. Smith, A. H. Winter, J. Am. Chem. Soc. 2015, 137, 3783–3786;
- 1hD. P. Walton, D. A. Dougherty, J. Am. Chem. Soc. 2017, 139, 4655–4658;
- 1iH. Zhang, C. Aonbangkhen, E. V. Tarasovetc, E. R. Ballister, D. M. Chenoweth, M. A. Lampson, Nat. Chem. Biol. 2017, 13, 1096–1101;
- 1jG. Bassolino, C. Nançoz, Z. Thiel, E. Bois, E. Vauthey, P. Rivera-Fuentes, Chem. Sci. 2018, 9, 387–391;
- 1kA. Bardhan, A. Deiters, Curr. Opin. Struct. Biol. 2019, 57, 164–175.
- 2
- 2aM. A. Miranda, H. Garcia, Chem. Rev. 1994, 94, 1063–1089;
- 2bS. Fukuzumi, K. Ohkubo, Org. Biomol. Chem. 2014, 12, 6059–6071;
- 2cJ. Xu, S. Shanmugam, C. Boyer, ACS Macro Lett. 2015, 4, 926–932;
- 2dN. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075–10166;
- 2eJ. Luo, J. Zhang, ACS Catal. 2016, 6, 873–877;
- 2fL. Wang, J. Byun, R. Li, W. Huang, K. A. I. Zhang, Adv. Synth. Catal. 2018, 360, 4312–4318.
- 3
- 3aL. D. Lavis, R. T. Raines, ACS Chem. Biol. 2008, 3, 142–155;
- 3bJ. Chan, S. C. Dodani, C. J. Chang, Nat. Chem. 2012, 4, 973–984;
- 3cJ. B. Grimm, L. M. Heckman, L. D. Lavis in Progress in Molecular Biology and Translational Science Vol. 113 (Ed.: ), Academic Press, Oxford, 2013, pp. 1–34;
- 3dE. A. Specht, E. Braselmann, A. E. Palmer, Annu. Rev. Physiol. 2017, 79, 93–117;
- 3eH. J. Knox, J. Chan, Acc. Chem. Res. 2018, 51, 2897–2905;
- 3fS. Husen Alamudi, Y.-T. Chang, Chem. Commun. 2018, 54, 13641–13653.
- 4Y. Huang, J. Xing, Q. Gong, L.-C. Chen, G. Liu, C. Yao, Z. Wang, H.-L. Zhang, Z. Chen, Q. Zhang, Nat. Commun. 2019, 10, 1–9.
- 5G.-J. Zhao, K.-L. Han, Acc. Chem. Res. 2012, 45, 404–413.
- 6
- 6aZ. R. Grabowski, J. Dobkowski, Pure Appl. Chem. 1983, 55, 245–252;
- 6bZ. R. Grabowski, K. Rotkiewicz, W. Rettig, Chem. Rev. 2003, 103, 3899–4032;
- 6cJ. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam, B. Z. Tang, Chem. Rev. 2015, 115, 11718–11940;
- 6dH. Qian, M. E. Cousins, E. H. Horak, A. Wakefield, M. D. Liptak, I. Aprahamian, Nat. Chem. 2017, 9, 83–87.
- 7C. Reichardt, Chem. Rev. 1994, 94, 2319–2358.
- 8
- 8aX. Song, A. Johnson, J. Foley, J. Am. Chem. Soc. 2008, 130, 17652–17653;
- 8bS. Singha, D. Kim, B. Roy, S. Sambasivan, H. Moon, A. S. Rao, J. Y. Kim, T. Joo, J. W. Park, Y. M. Rhee, T. Wang, K. H. Kim, Y. H. Shin, J. Jung, K. H. Ahn, Chem. Sci. 2015, 6, 4335–4342;
- 8cJ. B. Grimm, B. P. English, J. Chen, J. P. Slaughter, Z. Zhang, A. Revyakin, R. Patel, J. J. Macklin, D. Normanno, R. H. Singer, T. Lionnet, L. D. Lavis, Nat. Methods 2015, 12, 244–250;
- 8dX. Liu, Q. Qiao, W. Tian, W. Liu, J. Chen, M. J. Lang, Z. Xu, J. Am. Chem. Soc. 2016, 138, 6960–6963;
- 8eZ. Ye, W. Yang, C. Wang, Y. Zheng, W. Chi, X. Liu, Z. Huang, X. Li, Y. Xiao, J. Am. Chem. Soc. 2019, 141, 14491–14495;
- 8fA. N. Butkevich, M. L. Bossi, G. Lukinavičius, S. W. Hell, J. Am. Chem. Soc. 2019, 141, 981–989.
- 9
- 9aK. Imai, Y. Watanabe, Anal. Chim. Acta 1981, 130, 377–383;
- 9bS. Fery-Forgues, J.-P. Fayet, A. Lopez, J. Photochem. Photobiol. A 1993, 70, 229–243;
- 9cS. Uchiyama, T. Santa, K. Imai, J. Chem. Soc. Perkin Trans. 2 1999, 2525–2532.
- 10R. Afshar Ghotli, A. R. Abdul Aziz, I. M. Atadashi, D. B. Hasan, P. S. Kong, M. K. Aroua, J. Ind. Eng. Chem. 2015, 21, 1039–1043.
- 11
- 11aE. Lippert, Z. Naturforsch. A 1955, 10, 541–545;
- 11bN. Mataga, Y. Kaifu, M. Koizumi, Bull. Chem. Soc. Jpn. 1956, 29, 465–470.
- 12M. E. Jun, B. Roy, K. H. Ahn, Chem. Commun. 2011, 47, 7583–7601.
- 13G. V. Los, L. P. Encell, M. G. McDougall, D. D. Hartzell, N. Karassina, C. Zimprich, M. G. Wood, R. Learish, R. F. Ohana, M. Urh, D. Simpson, J. Mendez, K. Zimmerman, P. Otto, G. Vidugiris, J. Zhu, A. Darzins, D. H. Klaubert, R. F. Bulleit, K. V. Wood, ACS Chem. Biol. 2008, 3, 373–382.