Enantioselective Construction of Axially Chiral Amino Sulfide Vinyl Arenes by Chiral Sulfide-Catalyzed Electrophilic Carbothiolation of Alkynes
Yaoyu Liang
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorJieying Ji
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorXiaoyan Zhang
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorDr. Quanbin Jiang
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorJie Luo
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaodan Zhao
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorYaoyu Liang
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorJieying Ji
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorXiaoyan Zhang
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorDr. Quanbin Jiang
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorJie Luo
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaodan Zhao
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorGraphical Abstract
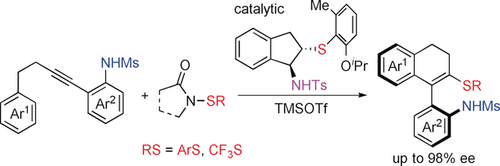
Enantioselective construction of axially chiral compounds by the electrophilic carbothiolation of alkynes is disclosed. This transformation is enabled by the use of a tosyl-protected bifunctional sulfide catalyst and mesyl-protected ortho-alkynylaryl amines. The obtained products can easily be converted into biaryl amino sulfides, biaryl amino sulfoxides, biaryl amines, vinyl–aryl amines, and other valuable difunctionalized compounds.
Abstract
The enantioselective construction of axially chiral compounds by electrophilic carbothiolation of alkynes is disclosed for the first time. This enantioselective transformation is enabled by the use of a Ts-protected bifunctional sulfide catalyst and Ms-protected ortho-alkynylaryl amines (Ts=tosyl; Ms=mesyl). Both electrophilic arylthiolating and electrophilic trifluoromethylthiolating reagents are suitable for this reaction. The obtained products of axially chiral vinyl–aryl amino sulfides can be easily converted into biaryl amino sulfides, biaryl amino sulfoxides, biaryl amines, vinyl–aryl amines, and other valuable difunctionalized compounds.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201915470-sup-0001-misc_information.pdf10.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aM. C. Kozlowski, B. J. Morgan, E. C. Linton, Chem. Soc. Rev. 2009, 38, 3193;
- 1bG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563;
- 1cA. Zask, J. Murphy, G. A. Ellestad, Chirality 2013, 25, 265.
- 2
- 2a Privileged chiral ligands and catalysts (Ed.: ), Wiley-VCH, Weinheim, 2011; For selected reviews, see:
- 2bY.-M. Li, F.-Y. Kwong, W.-Y. Yu, A. S. C. Chan, Coord. Chem. Rev. 2007, 251, 2119;
- 2cS. Schenker, A. Zamfir, M. Freund, S. B. Tsogoeva, Eur. J. Org. Chem. 2011, 2209.
- 3J. Kohno, Y. Koguchi, M. Nishio, K. Nakao, M. Kuroda, R. Shimizu, T. Ohnuki, S. Komatsubara, J. Org. Chem. 2000, 65, 990.
- 4For selected reviews, see:
- 4aP. J. Guiry, C. P. Saunders, Adv. Synth. Catal. 2004, 346, 497;
- 4bM. Mellah, A. Voituriez, E. Schulz, Chem. Rev. 2007, 107, 5133;
- 4cS. Otocka, M. Kwiatkowska, L. Madalińska, P. Kiełbasiński, Chem. Rev. 2017, 117, 4147.
- 5For selected examples, see:
- 5aS. Vyskočil, M. Smrčina, V. Hanuš, M. Polášek, P. Kočovský, J. Org. Chem. 1998, 63, 7738;
- 5bJ. J. Bürgi, R. Mariz, M. Gatti, E. Drinkel, X. Luan, S. Blumentritt, A. Linden, R. Dorta, Angew. Chem. Int. Ed. 2009, 48, 2768; Angew. Chem. 2009, 121, 2806;
- 5cT. Hoshi, K. Sasaki, S. Sato, Y. Ishii, T. Suzuki, H. Hagiwara, Org. Lett. 2011, 13, 932.
- 6
- 6aN. Vallavoju, S. Selvakumar, S. Jockusch, M. P. Sibi, J. Sivaguru, Angew. Chem. Int. Ed. 2014, 53, 5604; Angew. Chem. 2014, 126, 5710;
- 6bS. Liu, K. Maruoka, S. Shirakawa, Angew. Chem. Int. Ed. 2017, 56, 4819; Angew. Chem. 2017, 129, 4897.
- 7For selected reviews, see:
- 7aG. Bringmann, A. J. P. Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning, Angew. Chem. Int. Ed. 2005, 44, 5384; Angew. Chem. 2005, 117, 5518;
- 7bD. Zhang, Q. Wang, Coord. Chem. Rev. 2015, 286, 1;
- 7cJ. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418;
- 7dG. Ma, M. P. Sibi, Chem. Eur. J. 2015, 21, 11644;
- 7eY.-B. Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534.
- 8For selected examples, see:
- 8aA. Bermejo, A. Ros, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2008, 130, 15798;
- 8bX. Shen, G. O. Jones, D. A. Watson, B. Bhayana, S. L. Buchwald, J. Am. Chem. Soc. 2010, 132, 11278;
- 8cG. Xu, W. Fu, G. Liu, C. H. Senanayake, W. Tang, J. Am. Chem. Soc. 2014, 136, 570.
- 9For selected examples, see:
- 9aH. Egami, T. Katsuki, J. Am. Chem. Soc. 2009, 131, 6082;
- 9bL.-W. Qi, J.-H. Mao, J. Zhang, B. Tan, Nat. Chem. 2018, 10, 58;
- 9cL.-W. Qi, S. Li , S.-H. Xiang , J. Wang, B. Tan , Nat. Catal. 2019, 2, 314.
- 10For selected examples, see:
- 10aS. Shirakawa, X. Wu, K. Maruoka, Angew. Chem. Int. Ed. 2013, 52, 14200; Angew. Chem. 2013, 125, 14450;
- 10bK. Mori, Y. Ichikawa, M. Kobayashi, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 3964;
- 10cS. Lu, S. B. Poh, Y. Zhao, Angew. Chem. Int. Ed. 2014, 53, 11041; Angew. Chem. 2014, 126, 11221;
- 10dS.-C. Zheng, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2019, 58, 9215; Angew. Chem. 2019, 131, 9313.
- 11For selected examples, see:
- 11aT. Osako, Y. Uozumi, Org. Lett. 2014, 16, 5866;
- 11bR. J. Armstrong, M. D. Smith, Angew. Chem. Int. Ed. 2014, 53, 12822; Angew. Chem. 2014, 126, 13036.
- 12
- 12aF. Guo, L. C. Konkol, R. J. Thomson, J. Am. Chem. Soc. 2011, 133, 18;
- 12bV. S. Raut, M. Jean, N. Vanthuyne, C. Roussel, T. Constantieux, C. Bressy, X. Bugaut, D. Bonne, J. Rodriguez, J. Am. Chem. Soc. 2017, 139, 2140;
- 12cA. Link, C. Sparr, Angew. Chem. Int. Ed. 2018, 57, 7136; Angew. Chem. 2018, 130, 7254.
- 13
- 13aA. Link, C. Sparr, Angew. Chem. Int. Ed. 2014, 53, 5458; Angew. Chem. 2014, 126, 5562;
- 13bT. Shibata, A. Sekine, A. Mitake, K. S. Kanyiva, Angew. Chem. Int. Ed. 2018, 57, 15862; Angew. Chem. 2018, 130, 16088;
- 13cF. Xue, T. Hayashi, Angew. Chem. Int. Ed. 2018, 57, 10368; Angew. Chem. 2018, 130, 10525;
- 13dK. Xu, W. Li, S. Zhu, T. Zhu, Angew. Chem. Int. Ed. 2019, 58, 17625; Angew. Chem. 2019, 131, 17789.
- 14
- 14aK. Zhao, L. Duan, S. Xu, J. Jiang, Y. Fu, Z. Gu, Chem 2018, 4, 599;
- 14bM. Hou, R. Deng, Z. Gu, Org. Lett. 2018, 20, 5779;
- 14cQ. Li, M. Zhang, S. Zhan, Z. Gu, Org. Lett. 2019, 21, 6374;
- 14dK. Zhu, Q. Fang, Y. Wang, B. Tang, F. Zhang, ACS Catal. 2019, 9, 4951;
- 14eL. Duan, K. Zhao, Z. Wang, F.-L. Zhang, Z. Gu, ACS Catal. 2019, 9, 9852.
- 15
- 15aY.-H. Chen, L.-W. Qi, F. Fang, B. Tan, Angew. Chem. Int. Ed. 2017, 56, 16308; Angew. Chem. 2017, 129, 16526;
- 15bS. M. Maddox, G. A. Dawson, N. C. Rochester, A. B. Ayonon, C. E. Moore, A. L. Rheingold, J. L. Gustafson, ACS Catal. 2018, 8, 5443;
- 15cG. Liao, B. Li, H.-M. Chen, Q.-J. Yao, Y.-N. Xia, J. Luo, B.-F. Shi, Angew. Chem. Int. Ed. 2018, 57, 17151; Angew. Chem. 2018, 130, 17397.
- 16For the examples on catalytic enantioselective synthesis of axially chiral styrenes, see:
- 16aS.-C. Zheng, S. Wu, Q. Zhou, L. W. Chung, L. Ye, B. Tan, Nat. Commun. 2017, 8, 15238;
- 16bS. Jia, Z. Chen, N. Zhang, Y. Tan, Y. Liu, J. Deng, H. Yan, J. Am. Chem. Soc. 2018, 140, 7056;
- 16cY. Tan, S. Jia, F. Hu, Y. Liu, L. Peng, D. Li, H. Yan, J. Am. Chem. Soc. 2018, 140, 16893;
- 16dL. Peng, K. Li, C. Xie, S. Li, D. Xu, W. Qin, H. Yan, Angew. Chem. Int. Ed. 2019, 58, 17199; Angew. Chem. 2019, 131, 17359;
- 16eY.-B. Wang, P. Yu, Z.-P. Zhou, J. Zhang, J. Wang , S.-H. Luo, Q.-S. Gu , K. N. Houk, B. Tan, Nat. Catal. 2019, 2, 504; For the relevant highlight, see:
- 16fJ. Rodriguez, D. Bonne, Chem. Commun. 2019, 55, 11168.
- 17
- 17aJ. Feng, B. Li, Z. Gu, Angew. Chem. Int. Ed. 2016, 55, 2186; Angew. Chem. 2016, 128, 2226;
- 17bH. Wu, Z. S. Han, B. Qu, D. Wang, Y. Zhang, Y. Xu, N. Grinberg, H. Lee, J. J. Song, F. Roschangar, G. Wang, C. H. Senanayake, Adv. Synth. Catal. 2017, 359, 3927;
- 17cJ. D. Jolliffe, R. J. Armstrong, M. D. Smith, Nat. Chem. 2017, 9, 558.
- 18P. Chauhan, S. Mahajan, D. Enders, Chem. Rev. 2014, 114, 8807.
- 19
- 19aS. E. Denmark, D. J. P. Kornfilt, T. Vogler, J. Am. Chem. Soc. 2011, 133, 15308;
- 19bS. E. Denmark, A. Jaunet, J. Am. Chem. Soc. 2013, 135, 6419;
- 19cZ. Tao, K. A. Robb, K. Zhao, S. E. Denmark, J. Am. Chem. Soc. 2018, 140, 3569;
- 19dZ. Tao, K. A. Robb, J. L. Panger, S. E. Denmark, J. Am. Chem. Soc. 2018, 140, 15621;
- 19eA. Roth, S. E. Denmark, J. Am. Chem. Soc. 2019, 141, 13767;
- 19fA. Matviitsuk, S. E. Denmark, Angew. Chem. Int. Ed. 2019, 58, 12486; Angew. Chem. 2019, 131, 12616.
- 20
- 20aH. Guan, H. Wang, D. Huang, Y. Shi, Tetrahedron 2012, 68, 2728;
- 20bL. Li, Z. Li, D. Huang, H. Wang, Y. Shi, RSC Adv. 2013, 3, 4523.
- 21
- 21aH.-Y. Luo, Y.-Y. Xie, X.-F. Song, J.-W. Dong, D. Zhu, Z.-M. Chen, Chem. Commun. 2019, 55, 9367;
- 21bY.-Y. Xie, Z.-M. Chen, H.-Y. Luo, H. Shao, Y.-Q. Tu, X. Bao, R.-F. Cao, S.-Y. Zhang, J.-M. Tian, Angew. Chem. Int. Ed. 2019, 58, 12491; Angew. Chem. 2019, 131, 12621.
- 22
- 22aX. Liu, R. An, X. Zhang, J. Luo, X. Zhao, Angew. Chem. Int. Ed. 2016, 55, 5846; Angew. Chem. 2016, 128, 5940;
- 22bJ. Luo, Y. Liu, X. Zhao, Org. Lett. 2017, 19, 3434;
- 22cJ. Luo, X. Liu, X. Zhao, Synlett 2017, 28, 397;
- 22dJ. Luo, Q. Cao, X. Cao, X. Zhao, Nat. Commun. 2018, 9, 527;
- 22eX. Liu, Y. Liang, J. Ji, J. Luo, X. Zhao, J. Am. Chem. Soc. 2018, 140, 4782;
- 22fJ. Xu, Y. Zhang, T. Qin, X. Zhao, Org. Lett. 2018, 20, 6384;
- 22gT. Qin, Q. Jiang, J. Ji, J. Luo, X. Zhao, Org. Biomol. Chem. 2019, 17, 1763;
- 22hY. Liang, X. Zhao, ACS Catal. 2019, 9, 6896.
- 23
- 23aB.-L. Hu, S.-S. Pi, P.-C. Qian, J.-H. Li, X.-G. Zhang, J. Org. Chem. 2013, 78, 1300;
- 23bJ.-J. Wu, J. Xu, X. Zhao, Chem. Eur. J. 2016, 22, 15265;
- 23cX. Li, Y. Guo, Z. Shen, J. Org. Chem. 2018, 83, 2818;
- 23dZ. Zhang, P. He, H. Du, J. Xu, P. Li, J. Org. Chem. 2019, 84, 4517.
- 24
- 24aZ. Deng, J. Wei, L. Liao, H. Huang, X. Zhao, Org. Lett. 2015, 17, 1834;
- 24bL. Liao, R. Guo, X. Zhao, Angew. Chem. Int. Ed. 2017, 56, 3201; Angew. Chem. 2017, 129, 3249;
- 24cR. Guo, J. Huang, X. Zhao, ACS Catal. 2018, 8, 926.
- 25
- 25aF. Leroux, P. Jeschke, M. Schlosser, Chem. Rev. 2005, 105, 827;
- 25bK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881;
- 25cG. Landelle, A. Panossian, Curr. Top. Med. Chem. 2014, 14, 941.
- 26
- 26aP. Tian, H.-Q. Dong, G.-Q. Lin, ACS Catal. 2012, 2, 95;
- 26bH.-Q. Dong, M.-H. Xu, C.-G. Feng, X.-W. Sun, G.-Q. Lin, Org. Chem. Front. 2015, 2, 73.