Mechanical Force Induces Ylide-Free Cycloaddition of Nonscissible Aziridines
Sangmin Jung
Department of Chemistry, Korea University, Seoul, 02841 South Korea
Search for more papers by this authorCorresponding Author
Prof. Hyo Jae Yoon
Department of Chemistry, Korea University, Seoul, 02841 South Korea
Search for more papers by this authorSangmin Jung
Department of Chemistry, Korea University, Seoul, 02841 South Korea
Search for more papers by this authorCorresponding Author
Prof. Hyo Jae Yoon
Department of Chemistry, Korea University, Seoul, 02841 South Korea
Search for more papers by this authorGraphical Abstract
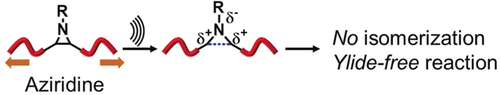
The force awakens: Mechanical-force-induced cycloaddition of intact aziridine groups in a macromolecule with dipolarophiles does not follow the reaction pathways that occur under traditional thermal and photochemical conditions. The aziridines do not undergo cis–trans isomerization, thus suggesting retention of the ring structure under force. This demonstrates that nonvulnerable chemical structures can be attractive mechanophores.
Abstract
The application of aziridines as nonvulnerable mechanophores is reported. Upon exposure to a mechanical force, stereochemically pure nonactivated aziridines incorporated into the backbone of a macromolecule do not undergo cis–trans isomerization, thus suggesting retention of the ring structure under force. Nonetheless, aziridines react with a dipolarophile and seem not to obey conventional reaction pathways that involve C−C or C−N bond cleavage prior to the cycloaddition. Our work demonstrates that a nonvulnerable chemical structure can be a mechanophore.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201915438-sup-0001-misc_information.pdf1.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. M. Caruso, D. A. Davis, Q. Shen, S. A. Odom, N. R. Sottos, S. R. White, J. S. Moore, Chem. Rev. 2009, 109, 5755–5798.
- 2
- 2aD. A. Davis, A. Hamilton, J. Yang, L. D. Cremar, D. V. Gough, S. L. Potisek, M. T. Ong, P. V. Braun, T. J. Martínez, S. R. White, J. S. Moore, N. R. Sottos, Nature 2009, 459, 68–72;
- 2bS. Akbulatov, Y. Tian, Z. Huang, T. J. Kucharski, Q.-Z. Yang, R. Boulatov, Science 2017, 357, 299–303;
- 2cJ. M. Lenhardt, M. T. Ong, R. Choe, C. R. Evenhuis, T. J. Martinez, S. L. Craig, Science 2010, 329, 1057–1060;
- 2dC. R. Hickenboth, J. S. Moore, S. R. White, N. R. Sottos, J. Baudry, S. R. Wilson, Nature 2007, 446, 423–427;
- 2eJ. M. Lenhardt, A. L. Black, S. L. Craig, J. Am. Chem. Soc. 2009, 131, 10818–10819;
- 2fH. M. Klukovich, Z. S. Kean, A. L. B. Ramirez, J. M. Lenhardt, J. X. Lin, X. Q. Hu, S. L. Craig, J. Am. Chem. Soc. 2012, 134, 9577–9580;
- 2gW. M. Huang, X. Wu, X. Gao, Y. F. Yu, H. Lei, Z. S. Zhu, Y. Shi, Y. L. Chen, M. Qin, W. Wang, Y. Cao, Nat. Chem. 2019, 11, 310–319.
- 3A. Piermattei, S. Karthikeyan, R. P. Sijbesma, Nat. Chem. 2009, 1, 133–137.
- 4Q. Wang, G. R. Gossweiler, S. L. Craig, X. Zhao, Nat. Commun. 2014, 5, 4899–4908.
- 5C. E. Diesendruck, L. Y. Zhu, J. S. Moore, Chem. Commun. 2014, 50, 13235–13238.
- 6
- 6aY. Ren, A. A. Banishev, K. S. Suslick, J. S. Moore, D. D. Dlott, J. Am. Chem. Soc. 2017, 139, 3974–3977;
- 6bM. Di Giannantonio, M. A. Ayer, E. Verde-Sesto, M. Lattuada, C. Weder, K. M. Fromm, Angew. Chem. Int. Ed. 2018, 57, 11445–11450; Angew. Chem. 2018, 130, 11616–11621.
- 7
- 7aA. L. B. Ramirez, Z. S. Kean, J. A. Orlicki, M. Champhekar, S. M. Elsakr, W. E. Krause, S. L. Craig, Nat. Chem. 2013, 5, 757–761;
- 7bJ. Wang, T. B. Kouznetsova, R. Boulatov, S. L. Craig, Nat. Commun. 2018, 7, 13433–13441;
- 7cX. Hu, M. E. McFadden, R. W. Barber, M. J. Robb, J. Am. Chem. Soc. 2018, 140, 14073–14077;
- 7dM. Zhang, G. D. Bo, J. Am. Chem. Soc. 2018, 140, 12724–12727.
- 8R. Stevenson, G. D. Bo, J. Am. Chem. Soc. 2017, 139, 16768–16771.
- 9Z. Chen, J. A. M. Mercer, X. Zhu, J. A. H. Romaniuk, R. Pfattner, L. Cegelski, T. J. Martinez, N. Z. Burns, Y. Xia, Science 2017, 357, 475–479.
- 10J. Wang, T. B. Kouznetsova, Z. Niu, M. T. Ong, H. M. Klukovich, A. L. Rheingold, T. J. Martinez, S. L. Craig, Nat. Chem. 2015, 7, 323–327.
- 11
- 11aK. Wei, Z. C. Gao, H. R. Liu, X. J. Wu, F. Wang, H. X. Xu, ACS Macro Lett. 2017, 6, 1146–1150;
- 11bY. Sha, Y. D. Zhang, E. H. Xu, Z. Wang, T. Y. Zhu, S. L. Craig, C. B. Tang, ACS Macro Lett. 2018, 7, 1174–1179;
- 11cB. Lee, Z. B. Niu, J. P. Wang, C. Slebodnick, S. L. Craig, J. Am. Chem. Soc. 2015, 137, 10826–10832.
- 12
- 12aJ. P. Wang, T. B. Kouznetsova, Z. B. Niu, A. L. Rheingold, S. L. Craig, J. Org. Chem. 2015, 80, 11895–11898;
- 12bJ. H. Yang, M. Horst, J. A. H. Romaniuk, Z. X. Jin, L. Cegelski, Y. Xia, J. Am. Chem. Soc. 2019, 141, 6479–6483;
- 12cM. E. McFadden, M. J. Robb, J. Am. Chem. Soc. 2019, 141, 11388–11392;
- 12dH. M. Klukovich, Z. S. Kean, S. T. Iacono, S. L. Craig, J. Am. Chem. Soc. 2011, 133, 17882–17888;
- 12eG. R. Gossweiler, G. B. Hewage, G. Soriano, Q. M. Wang, G. W. Welshofer, X. H. Zhao, S. L. Craig, ACS Macro Lett. 2014, 3, 216–219.
- 13M. Fedoryński, Chem. Rev. 2003, 103, 1099–1132.
- 14R. E. Parker, N. S. Isaacs, Chem. Rev. 1959, 59, 737–799.
- 15
- 15aJ. P. Bell, J. Appl. Polym. 1970, 14, 1901–1906;
- 15bI. T. Smith, Polymer 1961, 2, 95–108;
- 15cM. J. Tozer, T. F. Herpin, Tetrahedron 1996, 52, 8619–8683;
- 15dD. L. S. Brahms, W. P. Dailey, Chem. Rev. 1996, 96, 1585–1632;
- 15eI. H. Wani, S. H. M. J. logoad, J. Warna, A. Hayat, H. Li, V. A. Shukla, A. Orthaber, A. Grigoriev, R. Ahuja, K. Leifer, Nanoscale 2019, 11, 6571–6575;
- 15fS. Kang, H. K. Moon, H. J. Yoon, Macromolecules 2018, 51, 4068–4076.
- 16
- 16aS. Jung, S. Kang, J. Kuwabara, H. J. Yoon, Polym. Chem. 2019, 10, 4506–4512;
- 16bH. K. Moon, S. Kang, H. J. Yoon, Polym. Chem. 2017, 8, 2287–2291;
- 16cH. J. Jang, J. T. Lee, H. J. Yoon, Polym. Chem. 2015, 6, 3387–3391;
- 16dH. J. Yoon, Y. W. Kim, B. K. Lee, W. K. Lee, Y. Kim, H. J. Ha, Chem. Commun. 2007, 79–81.
- 17
- 17aT. Hashimoto, K. Maruoka, Chem. Rev. 2015, 115, 5366–5412;
- 17bG. Pandey, P. Banerjee, S. R. Gadre, Chem. Rev. 2006, 106, 4484–4517;
- 17cL. M. Stanley, M. P. Sibi, Chem. Rev. 2008, 108, 2887–2902.
- 18P. Dauban, G. Malik, Angew. Chem. Int. Ed. 2009, 48, 9026–9029; Angew. Chem. 2009, 121, 9188–9191.
- 19P. F. Lu, Tetrahedron 2010, 66, 2549–2560.
- 20M. Shipman, Synlett 2006, 3205–3217.
- 21
- 21aR. Huisgen, W. Scheer, H. Huber, J. Am. Chem. Soc. 1967, 89, 1753–1755;
- 21bP. J. S. Gomes, C. M. Nunes, A. A. C. C. Pais, T. M. V. D. P. E. Melo, L. G. Arnaut, Tetrahedron Lett. 2006, 47, 5475–5479;
- 21cA. L. Cardoso, T. M. V. D. P. E. Melo, Eur. J. Org. Chem. 2012, 6479–6501.
- 22M. K. Beyer, J. Chem. Phys. 2000, 112, 7307–7312.
- 23Z. Chen, X. Zhu, J. Yang, J. A. M. Mercer, N. Z. Burns, T. J. Martinez, Y. Xia, Nat. Chem. 2020, https://doi.org/10.1038/s41557-019-0396-0395.