Iron-Catalysed Radical Polymerisation by Living Bacteria
Mechelle R. Bennett
Division of Regenerative Medicine and Cellular Therapies, School of Pharmacy, University of Nottingham, University Park Campus, Nottingham, NG72RD UK
Search for more papers by this authorDr. Pratik Gurnani
Division of Molecular Therapeutics and Formulation, School of Pharmacy, University of Nottingham, University Park Campus, Nottingham, NG7 2RD UK
Search for more papers by this authorProf. Phil J. Hill
Division of Microbiology, Brewing and Biotechnology, School of Biosciences, University of Nottingham, Sutton Bonington Campus, Nottingham, LE12 5RD UK
Search for more papers by this authorProf. Cameron Alexander
Division of Molecular Therapeutics and Formulation, School of Pharmacy, University of Nottingham, University Park Campus, Nottingham, NG7 2RD UK
Search for more papers by this authorCorresponding Author
Prof. Frankie J. Rawson
Division of Regenerative Medicine and Cellular Therapies, School of Pharmacy, University of Nottingham, University Park Campus, Nottingham, NG72RD UK
Search for more papers by this authorMechelle R. Bennett
Division of Regenerative Medicine and Cellular Therapies, School of Pharmacy, University of Nottingham, University Park Campus, Nottingham, NG72RD UK
Search for more papers by this authorDr. Pratik Gurnani
Division of Molecular Therapeutics and Formulation, School of Pharmacy, University of Nottingham, University Park Campus, Nottingham, NG7 2RD UK
Search for more papers by this authorProf. Phil J. Hill
Division of Microbiology, Brewing and Biotechnology, School of Biosciences, University of Nottingham, Sutton Bonington Campus, Nottingham, LE12 5RD UK
Search for more papers by this authorProf. Cameron Alexander
Division of Molecular Therapeutics and Formulation, School of Pharmacy, University of Nottingham, University Park Campus, Nottingham, NG7 2RD UK
Search for more papers by this authorCorresponding Author
Prof. Frankie J. Rawson
Division of Regenerative Medicine and Cellular Therapies, School of Pharmacy, University of Nottingham, University Park Campus, Nottingham, NG72RD UK
Search for more papers by this authorGraphical Abstract
Abstract
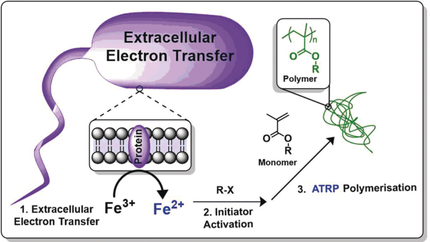
The ability to harness cellular redox processes for abiotic synthesis might allow the preparation of engineered hybrid living systems. Towards this goal we describe a new bacteria-mediated iron-catalysed reversible deactivation radical polymerisation (RDRP), with a range of metal-chelating agents and monomers that can be used under ambient conditions with a bacterial redox initiation step to generate polymers. Cupriavidus metallidurans, Escherichia coli, and Clostridium sporogenes species were chosen for their redox enzyme systems and evaluated for their ability to induce polymer formation. Parameters including cell and catalyst concentration, initiator species, and monomer type were investigated. Water-soluble synthetic polymers were produced in the presence of the bacteria with full preservation of cell viability. This method provides a means by which bacterial redox systems can be exploited to generate “unnatural” polymers in the presence of “host” cells, thus setting up the possibility of making natural–synthetic hybrid structures and conjugates.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201915084-sup-0001-misc_information.pdf2.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. F. Lutz, Macromol. Rapid Commun. 2017, 38, 1700582;
- 1bS. Celasun, D. Remmler, T. Schwaar, M. G. Weller, F. Du Prez, H. G. Börner, Angew. Chem. Int. Ed. 2019, 58, 1960–1964; Angew. Chem. 2019, 131, 1980–1984;
- 1cJ. O. Holloway, C. Mertens, F. E. Du Prez, N. Badi, Macromol. Rapid Commun. 2019, 40, 1800685;
- 1dY. Kametani, M. Sawamoto, M. Ouchi, Angew. Chem. Int. Ed. 2018, 57, 10905–10909; Angew. Chem. 2018, 130, 11071–11075;
- 1eM. Ouchi, M. Sawamoto, Polym. J. 2018, 50, 83–94;
- 1fG. Gody, P. B. Zetterlund, S. Perrier, S. Harrisson, Nat. Commun. 2016, 7, 10514;
- 1gN. G. Engelis, A. Anastasaki, G. Nurumbetov, N. P. Truong, V. Nikolaou, A. Shegiwal, M. R. Whittaker, T. P. Davis, D. M. Haddleton, Nat. Chem. 2017, 9, 171–178;
- 1hJ. F. Lutz, M. Ouchi, D. R. Liu, M. Sawamoto, Science 2013, 341, 1238149.
- 2
- 2aR. Szweda, M. Tschopp, O. Felix, G. Decher, J. F. Lutz, Angew. Chem. Int. Ed. 2018, 57, 15817–15821; Angew. Chem. 2018, 130, 16043–16047;
- 2bG. Cavallo, S. Poyer, J. A. Amalian, F. Dufour, A. Burel, C. Carapito, L. Charles, J. F. Lutz, Angew. Chem. Int. Ed. 2018, 57, 6266–6269; Angew. Chem. 2018, 130, 6374–6377;
- 2cS. Martens, A. Landuyt, P. Espeel, B. Devreese, P. Dawyndt, F. Du Prez, Nat. Commun. 2018, 9, 4451.
- 3D. Karamessini, T. Simon-Yarza, S. Poyer, E. Konishcheva, L. Charles, D. Letourneur, J. F. Lutz, Angew. Chem. Int. Ed. 2018, 57, 10574–10578; Angew. Chem. 2018, 130, 10734–10738.
- 4A. Kuroki, P. Sangwan, Y. Qu, R. Peltier, C. Sanchez-Cano, J. Moat, C. G. Dowson, E. G. L. Williams, K. E. S. Locock, M. Hartlieb, S. Perrier, ACS Appl. Mater. Interfaces 2017, 9, 40117–40126.
- 5
- 5aP. Q. Nguyen, N. M. D. Courchesne, A. Duraj-Thatte, P. Praveschotinunt, N. S. Joshi, Adv. Mater. 2018, 30, 1704847;
- 5bK. Pardee, S. Slomovic, P. Q. Nguyen, J. W. Lee, N. Donghia, D. Burrill, T. Ferrante, F. R. McSorley, Y. Furuta, A. Vernet, M. Lewandowski, C. N. Boddy, N. S. Joshi, J. J. Collins, Cell 2016, 167, 248–259.
- 6
- 6aC. Santoro, C. Arbizzani, B. Erable, I. Ieropoulos, J. Power Sources 2017, 356, 225–244;
- 6bI. Llorens, G. Untereiner, D. Jaillard, B. Gouget, V. Chapon, M. Carriere, PloS one 2012, 7, e51783;
- 6cH. Yokoyama, M. Ishida, T. Yamashita, J. Microbiol. Biotechnol. 2016, 26, 757–762;
- 6dO. Choi, B.-I. Sang, Biotechnol. Biofuels 2016, 9, 11.
- 7N. Bansal, J. J. Coetzee, E. M. N. Chirwa, Ecotoxicol. Environ. Saf. 2019, 172, 281–289.
- 8
- 8aX. Yun, Q. Zhang, M. Lv, H. Deng, Z. Deng, Y. Yu, Org. Biomol. Chem. 2019, 17, 454–460;
- 8bY. Wang, Y. Tashiro, K. Sonomoto, J. Biosci. Bioeng. 2015, 119, 10–18;
- 8cS. Slomovic, K. Pardee, J. J. Collins, Proc. Natl. Acad. Sci. USA 2015, 112, 14429–14435;
- 8dG. M. Church, M. B. Elowitz, C. D. Smolke, C. A. Voigt, R. Weiss, Nat. Rev. Mol. Cell Biol. 2014, 15, 289.
- 9
- 9aE. P. Magennis, F. Fernandez-Trillo, C. Sui, S. G. Spain, D. J. Bradshaw, D. Churchley, G. Mantovani, K. Winzer, C. Alexander, Nat. Mater. 2014, 13, 748–755;
- 9bJ. Geng, W. Li, Y. Zhang, N. Thottappillil, J. Clavadetscher, A. Lilienkampf, M. Bradley, Nat. Chem. 2019, 11, 578.
- 10J. Niu, D. J. Lunn, A. Pusuluri, J. I. Yoo, M. A. O'Malley, S. Mitragotri, H. T. Soh, C. J. Hawker, Nat. Chem. 2017, 9, 537–545.
- 11Y. Zhao, X. Zhang, Y. Wang, Z. Wu, J. An, Z. Lu, L. Mei, C. Li, Carbohydr. Polym. 2014, 105, 63–69.
- 12N. Idil, B. Mattiasson, Sensors 2017, 17, 708.
- 13
- 13aR.-B. Song, Y. Wu, Z.-Q. Lin, J. Xie, C. H. Tan, J. S. C. Loo, B. Cao, J.-R. Zhang, J.-J. Zhu, Q. Zhang, Angew. Chem. Int. Ed. 2017, 56, 10516–10520; Angew. Chem. 2017, 129, 10652–10656;
- 13bA. Ramanavicius, E. Andriukonis, A. Stirke, L. Mikoliunaite, Z. Balevicius, A. Ramanaviciene, Enzyme Microb. Technol. 2016, 83, 40–47;
- 13cE. Andriukonis, A. Stirke, A. Garbaras, L. Mikoliunaite, A. Ramanaviciene, V. Remeikis, B. Thornton, A. Ramanavicius, Colloids Surf. B 2018, 164, 224–231;
- 13dH. G. Sherman, J. M. Hicks, A. Jain, J. J. Titman, C. Alexander, S. Stolnik, F. J. Rawson, ChemBioChem 2019, 20, 1008–1013.
- 14
- 14aR. M. Apetrei, G. Carac, A. Ramanaviciene, G. Bahrim, C. Tanase, A. Ramanavicius, Colloids Surf. B 2019, 175, 671–679;
- 14bR. M. Apetrei, G. Carac, G. Bahrim, A. Ramanaviciene, A. Ramanavicius, Bioelectrochemistry 2018, 121, 46–55.
- 15
- 15aH. F. Downie, J. P. Standerwick, L. Burgess, L. S. Natrajan, J. R. Lloyd, Res. Microbiol. 2018, 169, 582–589;
- 15bJ. S. Gescher, C. D. Cordova, A. M. Spormann, Mol. Microbiol. 2008, 68, 706–719;
- 15cM. J. Colombo, J. Ha, J. R. Reinfelder, T. Barkay, N. Yee, Chem. Geol. 2014, 363, 334–340.
- 16
- 16aZ. Xue, D. He, X. Xie, Polym. Chem. 2015, 6, 1660–1687;
- 16bW. He, L. Zhang, J. Miao, Z. Cheng, X. Zhu, Macromol. Rapid Commun. 2012, 33, 1067–1073.
- 17
- 17aS. Averick, A. Simakova, S. Park, D. Konkolewicz, A. J. D. Magenau, R. A. Mehl, K. Matyjaszewski, ACS Macro Lett. 2012, 1, 6–10;
- 17bW. Tang, Y. Kwak, W. Braunecker, N. V. Tsarevsky, M. L. Coote, K. Matyjaszewski, J. Am. Chem. Soc. 2008, 130, 10702–10713.
- 18N. Kadayıfçıoğlu, H. Y. Acar, Eur. Polym. J. 2013, 49, 3366–3376.
- 19P. Monsieurs, H. Moors, R. Van Houdt, P. J. Janssen, A. Janssen, I. Coninx, M. Mergeay, N. Leys, Biometals 2011, 24, 1133–1151.
- 20L. Gao, X. Lu, H. Liu, J. Li, W. Li, R. Song, R. Wang, D. Zhang, J. Zhu, Front. Microbiol. 2019, 10, 575.
- 21
- 21aA. Pinochet-Barros, J. D. Helmann, Antioxid. Redox Signaling 2018, 29, 1858–1871;
- 21bK. D. Krewulak, H. J. Vogel, Biochim. Biophys. Acta Biomembr. 2008, 1778, 1781–1804.
- 22T. S. Magnuson, N. Isoyama, A. L. Hodges-Myerson, G. Davidson, M. J. Maroney, G. G. Geesey, D. R. Lovley, Biochem. J. 2001, 359, 147–152.
- 23M. L. Guerinot, Annu. Rev. Microbiol. 1994, 48, 743–772.