Branching Regulation in Olefin Polymerization via Lewis Acid Triggered Isomerization of Monomers
Graphical Abstract
Abstract
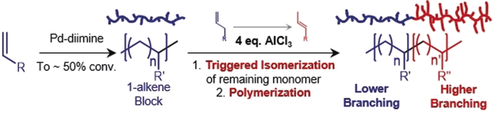
We present a new strategy to regulate branching in chain-walking olefin polymerization by triggering a rapid isomerization of 1-alkene monomers into internal olefins by adding a Lewis acid. Polymerization of internal alkenes proceeds via chain-walking to give polymers with much higher branching than 1-alkene analogues. The utility of this approach is exemplified by synthesis of well-defined block copolymers with distinct branching characteristics per block by addition of Lewis acid midway through a reaction. We propose a novel mechanism whereby Lewis acid undergoes a counterion swap with the complex which favors isomerization as well as forming adducts with ancillary ligands, freeing coordination sites for internal alkene coordination polymerization.
Conflict of interest
The authors declare no conflict of interest.