Biomimetic Hydrogenation Catalyzed by a Manganese Model of [Fe]-Hydrogenase
Dr. Hui-Jie Pan
Laboratory of Inorganic Synthesis and Catalysis, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), ISIC-LSCI, BCH 3305, Lausanne, 1015 Switzerland
Search for more papers by this authorCorresponding Author
Prof. Xile Hu
Laboratory of Inorganic Synthesis and Catalysis, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), ISIC-LSCI, BCH 3305, Lausanne, 1015 Switzerland
Search for more papers by this authorDr. Hui-Jie Pan
Laboratory of Inorganic Synthesis and Catalysis, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), ISIC-LSCI, BCH 3305, Lausanne, 1015 Switzerland
Search for more papers by this authorCorresponding Author
Prof. Xile Hu
Laboratory of Inorganic Synthesis and Catalysis, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), ISIC-LSCI, BCH 3305, Lausanne, 1015 Switzerland
Search for more papers by this authorGraphical Abstract
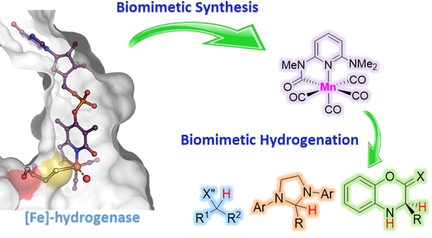
The design and development of a manganese(I) mimic of [Fe]-hydrogenase is reported. This complex exhibits the highest activity and broadest scope in catalytic hydrogenation among known mimics. Thanks to its biomimetic nature, the complex exhibits unique activity in the hydrogenation of compounds analogous to methenyl-H4MPT+, the natural substrate of [Fe]-hydrogenase.
Abstract
[Fe]-hydrogenase is an efficient biological hydrogenation catalyst. Despite intense research, Fe complexes mimicking the active site of [Fe]-hydrogenase have not achieved turnovers in hydrogenation reactions. Herein, we describe the design and development of a manganese(I) mimic of [Fe]-hydrogenase. This complex exhibits the highest activity and broadest scope in catalytic hydrogenation among known mimics. Thanks to its biomimetic nature, the complex exhibits unique activity in the hydrogenation of compounds analogous to methenyl-H4MPT+, the natural substrate of [Fe]-hydrogenase. This activity enables asymmetric relay hydrogenation of benzoxazinones and benzoxazines, involving the hydrogenation of a chiral hydride transfer agent using our catalyst coupled to Lewis acid-catalyzed hydride transfer from this agent to the substrates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201914377-sup-0001-misc_information.pdf6.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Noyori, T. Ohkuma, Angew. Chem. Int. Ed. 2001, 40, 40–73; Angew. Chem. 2001, 113, 40–75;
- 1bW. S. Knowles, Angew. Chem. Int. Ed. 2002, 41, 1998; Angew. Chem. 2002, 114, 2096;
- 1cR. Noyori, Angew. Chem. Int. Ed. 2002, 41, 2008–2022;
10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2108–2123;
- 1dJ. P. Genet, Acc. Chem. Res. 2003, 36, 908.
- 2
- 2aT. Zell, D. Milstein, Acc. Chem. Res. 2015, 48, 1979–1994;
- 2bR. H. Morris, Acc. Chem. Res. 2015, 48, 1494–1502;
- 2cM. Garbe, K. Junge, M. Beller, Eur. J. Org. Chem. 2017, 4344–4362;
- 2dG. A. Filonenko, R. van Putten, E. J. M. Hensen, E. A. Pidko, Chem. Soc. Rev. 2018, 47, 1459–1483;
- 2eF. Kallmeier, R. Kempe, Angew. Chem. Int. Ed. 2018, 57, 46–60; Angew. Chem. 2018, 130, 48–63;
- 2fL. Alig, M. Fritz, S. Schneider, Chem. Rev. 2019, 119, 2681–2751.
- 3S. Shima, U. Ermler, Eur. J. Inorg. Chem. 2011, 963–972.
- 4G. Huang, T. Wagner, M. D. Wodrich, K. Ataka, E. Bill, U. Ermler, X. Hu, S. Shima, Nat. Catal. 2019, 2, 537–543.
- 5
- 5aS. Shima, O. Pilak, S. Vogt, M. Schick, M. S. Stagni, W. Meyer-Klaucke, E. Warkentin, R. K. Thauer, U. Ermler, Science 2008, 321, 572–575;
- 5bT. Hiromoto, K. Ataka, O. Pilak, S. Vogt, M. S. Stagni, W. Meyer-Klaucke, E. Warkentin, R. K. Thauer, S. Shima, U. Ermler, FEBS Lett. 2009, 583, 585–590.
- 6S. Shima, D. Chen, T. Xu, M. D. Wodrich, T. Fujishiro, K. M. Schultz, J. Kahnt, K. Ataka, X. Hu, Nat. Chem. 2015, 7, 995–1002.
- 7
- 7aD. Schilter, J. M. Camara, M. T. Huynh, S. Hammes-Schiffer, T. B. Rauchfuss, Chem. Rev. 2016, 116, 8693–8749;
- 7bK. M. Schultz, D. Chen, X. Hu, Chem. Asian J. 2013, 8, 1068–1075;
- 7cP. J. Turrell, J. A. Wright, J. N. T. Peck, V. S. Oganesyan, C. J. Pickett, Angew. Chem. Int. Ed. 2010, 49, 7508–7511; Angew. Chem. 2010, 122, 7670–7673;
- 7dD. Chen, R. Scopelliti, X. Hu, Angew. Chem. Int. Ed. 2011, 50, 5671–5673; Angew. Chem. 2011, 123, 5789–5791;
- 7eD. Chen, R. Scopelliti, X. Hu, Angew. Chem. Int. Ed. 2012, 51, 1919–1921; Angew. Chem. 2012, 124, 1955–1957;
- 7fB. Hu, D. Chen, X. Hu, Chem. Eur. J. 2014, 20, 1677–1682;
- 7gT. Xu, C.-J. M. Yin, M. D. Wodrich, S. Mazza, K. M. Schultz, R. Scopelliti, X. Hu, J. Am. Chem. Soc. 2016, 138, 3270–3273;
- 7hJ. Seo, T. A. Manes, M. J. Rose, Nat. Chem. 2017, 9, 552–557;
- 7iS. A. Kerns, A. C. Magtaan, P. R. Vong, M. J. Rose, Angew. Chem. Int. Ed. 2018, 57, 2855–2858; Angew. Chem. 2018, 130, 2905–2908;
- 7jY. I. Cho, G. Durgaprasad, M. J. Rose, Inorg. Chem. 2019, 58, 12689–12699.
- 8K. F. Kalz, A. Brinkmeier, S. Dechert, R. A. Mata, F. Meyer, J. Am. Chem. Soc. 2014, 136, 16626–16634.
- 9H.-J. Pan, G. Huang, M. D. Wodrich, F. F. Tirani, K. Ataka, S. Shima, X. Hu, Nat. Chem. 2019, 11, 669–675.
- 10
- 10aD. S. Mérel, M. L. T. Do, S. Gaillard, P. Dupau, J.-L. Renaud, Coord. Chem. Rev. 2015, 288, 50–68;
- 10bS. Chakraborty, P. Bhattacharya, H. Dai, H. Guan, Acc. Chem. Res. 2015, 48, 1995–2003;
- 10cC. S. G. Seo, R. H. Morris, Organometallics 2019, 38, 47–65.
- 11R. van Putten, E. A. Uslamin, M. Garbe, C. Liu, A. Gonzalez-de-Castro, M. Lutz, K. Junge, E. J. M. Hensen, M. Beller, L. Lefort, E. A. Pidko, Angew. Chem. Int. Ed. 2017, 56, 7531–7534; Angew. Chem. 2017, 129, 7639–7642.
- 12S. Elangovan, C. Topf, S. Fischer, H. Jiao, A. Spannenberg, W. Baumann, R. Ludwig, K. Junge, M. Beller, J. Am. Chem. Soc. 2016, 138, 8809–8814.
- 13M. Hatazawa, N. Yoshie, H. Seino, Inorg. Chem. 2017, 56, 8087–8099.
- 14L. Bai, T. Fujishiro, G. Huang, J. Koch, A. Takabayashi, M. Yokono, A. Tanaka, T. Xu, X. Hu, U. Ermler, S. Shima, Faraday Discuss. 2017, 198, 37–58.
- 15
- 15aH. Lin, Org. Biomol. Chem. 2007, 5, 2541–2554;
- 15bW. Ying, Antioxid. Redox Signaling 2008, 10, 179–206;
- 15cR. H. Houtkooper, C. Cantó, R. J. Wanders, J. Auwerx, Endocr. Rev. 2010, 31, 194–223.
- 16J. Wang, Z.-H. Zhu, M.-W. Chen, Q.-A. Chen, Y.-G. Zhou, Angew. Chem. Int. Ed. 2019, 58, 1813–1817; Angew. Chem. 2019, 131, 1827–1831.
- 17
- 17aM. Rueping, A. P. Antonchick, T. Theissmann, Angew. Chem. Int. Ed. 2006, 45, 6751–6755; Angew. Chem. 2006, 118, 6903–6907;
- 17bR. I. Storer, D. E. Carrera, Y. Ni, D. W. C. MacMillan, J. Am. Chem. Soc. 2006, 128, 84–86.
- 18
- 18aJ. Ilaš, P. Š. Anderluh, M. S. Dolenc, D. Kikelj, Tetrahedron 2005, 61, 7325–7348;
- 18bF. A. Macías, D. Marín, A. Oliveros-Bastidas, J. M. G. Molinillo, Nat. Prod. Rep. 2009, 26, 478–489.
- 19
- 19aQ.-A. Chen, M.-W. Chen, C.-B. Yu, L. Shi, D.-S. Wang, Y. Yang, Y.-G. Zhou, J. Am. Chem. Soc. 2011, 133, 16432–16435;
- 19bQ.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang, Y.-G. Zhou, J. Am. Chem. Soc. 2012, 134, 2442–2448;
- 19cL.-Q. Lu, Y. Li, K. Junge, M. Beller, J. Am. Chem. Soc. 2015, 137, 2763–2768;
- 19dL. Zhao, J. Wei, J. Lu, C. He, C. Duan, Angew. Chem. Int. Ed. 2017, 56, 8692–8696; Angew. Chem. 2017, 129, 8818–8822.
- 20CCDC 1958229 contains the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.