Optimal Geometrical Configuration of Cobalt Cations in Spinel Oxides to Promote Oxygen Evolution Reaction
Graphical Abstract
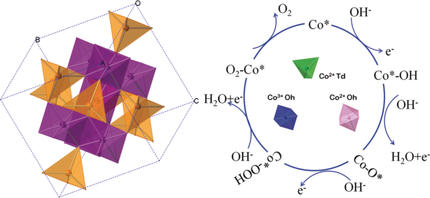
MgCo2O4, CoCr2O4, and Co2TiO4 were selected, where only Co3+ in the center of octahedron (Oh), Co2+ in the center of tetrahedron (Td), and Co2+ in the center of Oh can be active sites as model electrocatalysts for the oxygen evolution reaction. Co3+(Oh) sites are the best geometrical configuration; Co2+(Oh) sites exhibit better electrochemical activity than Co2+(Td).
Abstract
MgCo2O4, CoCr2O4, and Co2TiO4 were selected, where only Co3+ in the center of octahedron (Oh), Co2+ in the center of tetrahedron (Td), and Co2+ in the center of Oh, can be active sites for the oxygen evolution reaction (OER). Co3+(Oh) sites are the best geometrical configuration for OER. Co2+(Oh) sites exhibit better activity than Co2+(Td). Calculations demonstrate the conversion of O* into OOH* is the rate-determining step for Co3+(Oh) and Co2+(Td). For Co2+(Oh), it is thermodynamically favorable for the formation of OOH* but difficult for the desorption of O2. Co3+(Oh) needs to increase the lowest Gibbs free energy over Co2+(Oh) and Co2+(Td), which contributes to the best activity. The coexistence of Co3+(Oh) and Co2+(Td) in Co3O4 can promote the formation of OOH* and decrease the free-energy barrier. This work screens out the optimal geometrical configuration of cobalt cations for OER and gives a valuable principle to design efficient electrocatalysts.
Conflict of interest
The authors declare no conflict of interest.