Barcoding Biological Reactions with DNA-Functionalized Vesicles
Justin A. Peruzzi
Department of Chemical and Biological Engineering, Northwestern University, USA
Search for more papers by this authorMiranda L. Jacobs
Department of Biomedical Engineering, Northwestern University, McCormick School of Engineering, Technological Institute, 2145 Sheridan Road, Evanston, Il, 60208 USA
Search for more papers by this authorTimothy Q. Vu
Department of Biomedical Engineering, Northwestern University, McCormick School of Engineering, Technological Institute, 2145 Sheridan Road, Evanston, Il, 60208 USA
Search for more papers by this authorKenneth S. Wang
Department of Biomedical Engineering, Northwestern University, McCormick School of Engineering, Technological Institute, 2145 Sheridan Road, Evanston, Il, 60208 USA
Search for more papers by this authorCorresponding Author
Prof. Neha P. Kamat
Department of Biomedical Engineering, Northwestern University, McCormick School of Engineering, Technological Institute, 2145 Sheridan Road, Evanston, Il, 60208 USA
Center for Synthetic Biology, Northwestern University, USA
Chemistry of Life Processes Institute, Northwestern University, USA
Search for more papers by this authorJustin A. Peruzzi
Department of Chemical and Biological Engineering, Northwestern University, USA
Search for more papers by this authorMiranda L. Jacobs
Department of Biomedical Engineering, Northwestern University, McCormick School of Engineering, Technological Institute, 2145 Sheridan Road, Evanston, Il, 60208 USA
Search for more papers by this authorTimothy Q. Vu
Department of Biomedical Engineering, Northwestern University, McCormick School of Engineering, Technological Institute, 2145 Sheridan Road, Evanston, Il, 60208 USA
Search for more papers by this authorKenneth S. Wang
Department of Biomedical Engineering, Northwestern University, McCormick School of Engineering, Technological Institute, 2145 Sheridan Road, Evanston, Il, 60208 USA
Search for more papers by this authorCorresponding Author
Prof. Neha P. Kamat
Department of Biomedical Engineering, Northwestern University, McCormick School of Engineering, Technological Institute, 2145 Sheridan Road, Evanston, Il, 60208 USA
Center for Synthetic Biology, Northwestern University, USA
Chemistry of Life Processes Institute, Northwestern University, USA
Search for more papers by this authorGraphical Abstract
Abstract
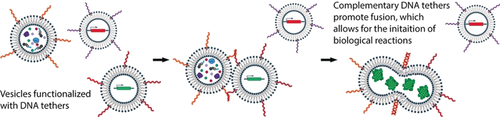
Targeted vesicle fusion is a promising approach to selectively control interactions between vesicle compartments and would enable the initiation of biological reactions in complex aqueous environments. Here, we explore how two features of vesicle membranes, DNA tethers and phase-segregated membranes, promote fusion between specific vesicle populations. Membrane phase-segregation provides an energetic driver for membrane fusion that increases the efficiency of DNA-mediated fusion events. The orthogonality provided by DNA tethers allows us to direct fusion and delivery of DNA cargo to specific vesicle populations. Vesicle fusion between DNA-tethered vesicles can be used to initiate in vitro protein expression to produce model soluble and membrane proteins. Engineering orthogonal fusion events between DNA-tethered vesicles provides a new strategy to control the spatiotemporal dynamics of cell-free reactions, expanding opportunities to engineer artificial cellular systems.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201911544-sup-0001-misc_information.pdf1.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Simons, G. Raposo, Curr. Opin. Cell Biol. 2009, 21, 575–581.
- 2E. Cocucci, J. Meldolesi, Trends Cell Biol. 2015, 25, 364–372.
- 3W. Lösche, T. Scholz, U. Temmler, V. Oberle, R. A. Claus, Platelets 2004, 15, 109–115.
- 4S. Sadallah, C. Eken, P. J. Martin, J. A. Schifferli, J. Immunol. 2011, 186, 6543–6552.
- 5F. Caschera, T. Sunami, T. Matsuura, H. Suzuki, M. M. Hanczyc, T. Yomo, Langmuir 2011, 27, 13082–13090.
- 6K. P. Adamala, D. A. Martin-Alarcon, K. R. Guthrie-Honea, E. S. Boyden, Nat. Chem. 2017, 9, 431–439.
- 7G. Bolognesi, M. S. Friddin, A. Salehi-Reyhani, N. E. Barlow, N. J. Brooks, O. Ces, Y. Elani, Nat. Commun. 2018, 9, 1882.
- 8G. Stengel, R. Zahn, F. Höök, J. Am. Chem. Soc. 2007, 129, 9584–9585.
- 9Y.-H. M. Chan, B. van Lengerich, S. G. Boxer, Biointerphases 2008, 3, FA 17–FA21.
- 10K. M. Flavier, S. G. Boxer, Biophys. J. 2017, 113, 1260–1268.
- 11Y.-H. M. Chan, B. van Lengerich, S. G. Boxer, Proc. Natl. Acad. Sci. USA 2009, 106, 979–984.
- 12B. van Lengerich, R. J. Rawle, S. G. Boxer, Langmuir 2010, 26, 8666–8672.
- 13Y.-H. M. Chan, P. Lenz, S. G. Boxer, Proc. Natl. Acad. Sci. USA 2007, 104, 18913–18918.
- 14G. Stengel, L. Simonsson, R. A. Campbell, F. Höök, J. Phys. Chem. B 2008, 112, 8264–8274.
- 15O. Ries, P. M. G. Löffler, S. Vogel, Org. Biomol. Chem. 2015, 13, 9673–9680.
- 16O. Ries, P. M. G. Löffler, A. Rabe, J. J. Malavan, S. Vogel, Org. Biomol. Chem. 2017, 15, 8936–8945.
- 17P. M. G. Löffler, O. Ries, A. Rabe, A. H. Okholm, R. P. Thomsen, J. Kjems, S. Vogel, Angew. Chem. Int. Ed. 2017, 56, 13228–13231; Angew. Chem. 2017, 129, 13410–13414.
- 18K. Simons, E. Ikonen, Nature 1997, 387, 569–572.
- 19R. Varma, S. Mayor, Nature 1998, 394, 798–801.
- 20D. M. Owen, D. J. Williamson, A. Magenau, K. Gaus, Nat. Commun. 2012, 3, 1256.
- 21W. H. Binder, V. Barragan, F. M. Menger, Angew. Chem. Int. Ed. 2003, 42, 5802–5827; Angew. Chem. 2003, 115, 5980–6007.
- 22H. J. Risselada, Biophys. J. 2017, 112, 2475–2478.
- 23D. E. Saslowsky, J. Lawrence, X. Ren, D. A. Brown, R. M. Henderson, J. Michael Edwardson, J. Biol. Chem. 2002, 277, 26966–26970.
- 24P. I. Kuzmin, S. A. Akimov, Y. A. Chizmadzhev, J. Zimmerberg, F. S. Cohen, Biophys. J. 2005, 88, 1120–1133.
- 25T. Baumgart, S. T. Hess, W. W. Webb, Nature 2003, 425, 821–824.
- 26S.-T. Yang, V. Kiessling, L. K. Tamm, Nat. Commun. 2016, 7, 11401.
- 27S. L. Veatch, S. L. Keller, Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 172–185.
- 28S.-T. Yang, V. Kiessling, J. A. Simmons, J. M. White, L. K. Tamm, Nat. Chem. Biol. 2015, 11, 424–431.
- 29Z. I. Imam, L. E. Kenyon, G. Ashby, F. Nagib, M. Mendicino, C. Zhao, A. K. Gadok, J. C. Stachowiak, Cell. Mol. Bioeng. 2017, 10, 387–403.
- 30M. E. Haque, B. R. Lentz, Biochemistry 2004, 43, 3507.
- 31M. E. Haque, T. J. Mcintosh, B. R. Lentz, Biochemistry 2001, 40, 4340.
- 32Z. Chen, R. P. Rand, Biophys. J. 1997, 73, 267–276.
- 33J. L. Rubenstein, B. A. Smith, H. M. McConnell, Proc. Natl. Acad. Sci. USA 1979, 76, 15–18.
- 34J. Zhang, R. Xue, W.-Y. Ong, P. Chen, Biophys. J. 2009, 97, 1371–1380.
- 35A. Linetti, A. Fratangeli, E. Taverna, P. Valnegri, M. Francolini, V. Cappello, M. Matteoli, M. Passafaro, P. Rosa, J. Cell Sci. 2010, 123, 595–605.
- 36C. R. Wasser, M. Ertunc, X. Liu, E. T. Kavalali, J. Physiol. 2007, 579, 413–429.
- 37B. S. Stratton, J. M. Warner, Z. Wu, J. Nikolaus, G. Wei, E. Wagnon, D. Baddeley, E. Karatekin, B. O'Shaughnessy, Biophys. J. 2016, 110, 1538–1550.
- 38J. Tong, P. P. Borbat, J. H. Freed, Y.-K. Shin, Proc. Natl. Acad. Sci. USA 2009, 106, 5141–5146.
- 39A. J. B. Kreutzberger, V. Kiessling, L. K. Tamm, Biophys. J. 2015, 109, 319–329.
- 40B. K.-K. Fung, L. Stryer, Biochemistry 1978, 17, 5241–5248.
- 41D. K. Struck, D. Hoekstra, R. E. Pagano, Biochemistry 1981, 20, 4093–4099.
- 42S.-T. Yang, A. J. B. Kreutzberger, V. Kiessling, B. K. Ganser-Pornillos, J. M. White, L. K. Tamm, Sci. Adv. 2017, 3, e1700338.
- 43D. Scherfeld, N. Kahya, P. Schwille, Biophys. J. 2003, 85, 3758–3768.
- 44J. Zhao, J. Wu, F. A. Heberle, T. T. Mills, P. Klawitter, G. Huang, G. Costanza, G. W. Feigenson, Biochim. Biophys. Acta Biomembr. 2007, 1768, 2764–2776.
- 45J. R. Silvius, in Lipid-Protein Interact., Wiley, New York, 1982.
- 46J. Dai, M. Alwarawrah, M. R. Ali, G. W. Feigenson, J. Huang, J. Phys. Chem. B 2011, 115, 1662–1671.
- 47F. A. Heberle, R. S. Petruzielo, J. Pan, P. Drazba, N. Kuč, R. F. Standaert, G. W. Feigenson, J. Katsaras, J. Am. Chem. Soc. 2013, 135, 6853–6859.
- 48A. J. García-Sáez, S. Chiantia, P. Schwille, J. Biol. Chem. 2007, 282, 33537–33544.
- 49V. A. J. Frolov, Y. A. Chizmadzhev, F. S. Cohen, J. Zimmerberg, Biophys. J. 2006, 91, 189–205.
- 50H. J. Risselada, G. Bubnis, H. Grubmüller, Proc. Natl. Acad. Sci. USA 2014, 111, 11043–11048.
- 51M. Chemin, P.-M. Brun, S. Lecommandoux, O. Sandre, J.-F. Le Meins, Soft Matter 2012, 8, 2867.
- 52T. P. T. Dao, F. Fernandes, M. Er-Rafik, R. Salva, M. Schmutz, A. Brûlet, M. Prieto, O. Sandre, J.-F. Le Meins, ACS Macro Lett. 2015, 4, 182–186.
- 53T. P. T. Dao, F. Fernandes, E. Ibarboure, K. Ferji, M. Prieto, O. Sandre, J.-F. Le Meins, Soft Matter 2017, 13, 627–637.
- 54J. Nam, P. A. Beales, T. K. Vanderlick, Langmuir 2011, 27, 1–6.
- 55J. Nam, T. K. Vanderlick, P. A. Beales, Soft Matter 2012, 8, 7982.
- 56E. Sezgin, I. Levental, M. Grzybek, G. Schwarzmann, V. Mueller, A. Honigmann, V. N. Belov, C. Eggeling, Ü. Coskun, K. Simons, et al., Biochim. Biophys. Acta Biomembr. 2012, 1818, 1777–1784.
- 57N. Kahya, D. Scherfeld, K. Bacia, B. Poolman, P. Schwille, J. Biol. Chem. 2003, 278, 28109–28115.
- 58E. P. Petrov, R. Petrosyan, P. Schwille, Soft Matter 2012, 8, 7552.
- 59J. C. Stachowiak, C. C. Hayden, D. Y. Sasaki, Proc. Natl. Acad. Sci. USA 2010, 107, 7781–7786.
- 60J. C. Stachowiak, C. C. Hayden, M. A. A. Sanchez, J. Wang, B. C. Bunker, J. A. Voigt, D. Y. Sasaki, Langmuir 2011, 27, 1457–1462.
- 61P. A. Beales, C. L. Bergstrom, N. Geerts, J. T. Groves, T. K. Vanderlick, Langmuir 2011, 27, 6107–6115.
- 62P. A. Beales, J. Nam, T. K. Vanderlick, Soft Matter 2011, 7, 1747–1755.
- 63M. Schulz, S. Werner, K. Bacia, W. H. Binder, Angew. Chem. Int. Ed. 2013, 52, 1829–1833; Angew. Chem. 2013, 125, 1877–1882.
- 64N. Momin, S. Lee, A. K. Gadok, D. J. Busch, G. D. Bachand, C. C. Hayden, J. C. Stachowiak, D. Y. Sasaki, Soft Matter 2015, 11, 3241–3250.
- 65K. Oglęcka, P. Rangamani, B. Liedberg, R. S. Kraut, A. N. Parikh, eLife 2014, 3, e03695.
- 66E. Sezgin, I. Levental, S. Mayor, C. Eggeling, Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374.
- 67C. Xu, S. Hu, X. Chen, Mater. Today 2016, 19, 516–532.
- 68B. C. Buddingh′, J. C. M. Van Hest, Acc. Chem. Res. 2017, 50, 769–777.
- 69J. W. Hindley, Y. Elani, C. M. McGilvery, S. Ali, C. L. Bevan, R. V. Law, O. Ces, Nat. Commun. 2018, 9, 1093.
- 70Y. Elani, R. V. Law, O. Ces, Nat. Commun. 2014, 5, 5305.
- 71T. Sunami, T. Matsuura, H. Suzuki, T. Yomo, in Cell-Free Protein Prod. Methods in Mol. Biol. Ed.: , Humana Press, 2010, 243–256.
- 72R. Marshall, V. Noireaux, Methods Mol. Biol. 2018, 1772, 61–93.
- 73R. Lentini, N. Yeh Martín, S. S. Mansy, Curr. Opin. Chem. Biol. 2016, 34, 53–61.
- 74C. E. Hilburger, M. L. Jacobs, K. R. Lewis, J. A. Peruzzi, N. P. Kamat, ACS Synth. Biol. 2019, 8, 1224–1230.
- 75R. Lentini, S. P. Santero, F. Chizzolini, D. Cecchi, J. Fontana, M. Marchioretto, C. Del Bianco, J. L. Terrell, A. C. Spencer, L. Martini, et al., Nat. Commun. 2014, 5, 4012.
- 76J. C. Stark, A. Huang, P. Q. Nguyen, R. S. Dubner, K. J. Hsu, T. C. Ferrante, M. Anderson, A. Kanapskyte, Q. Mucha, J. S. Packett, et al., Sci. Adv. 2018, 4, eaat 5107.
- 77J. Heureaux, D. Chen, V. L. Murray, C. X. Deng, A. P. Liu, Cell. Mol. Bioeng. 2014, 7, 307–319.
- 78M. L. Jacobs, M. A. Boyd, N. P. Kamat, Proc. Natl. Acad. Sci. USA 2019, 116, 4031–4036.