Biocatalytic Friedel–Crafts Alkylation Using a Promiscuous Biosynthetic Enzyme
Graphical Abstract
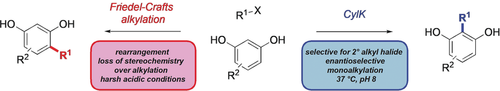
Enzymatic alkylation: The cylindrocyclophane biosynthetic enzyme CylK is identified as a promiscuous catalyst for Friedel–Crafts alkylation of resorcinol substrates with alkyl halide electrophiles. This transformation, which proceeds with exceptional regioselectivity and stereospecificity, highlights the promise of enzymatic catalysis for enabling mild and selective C−C bond-forming synthetic methodology.
Abstract
The Friedel–Crafts alkylation is commonly used in organic synthesis to form aryl–alkyl C−C linkages. However, this reaction lacks the stereospecificity and regiocontrol of enzymatic catalysis. Here, we describe a stereospecific, biocatalytic Friedel–Crafts alkylation of the 2-position of resorcinol rings using the cylindrocyclophane biosynthetic enzyme CylK. This regioselectivity is distinct from that of the classical Friedel–Crafts reaction. Numerous secondary alkyl halides are accepted by this enzyme, as are resorcinol rings with a variety of substitution patterns. Finally, we have been able to use this transformation to access novel analogues of the clinical drug candidate benvitimod that are challenging to construct with existing synthetic methods. These findings highlight the promise of enzymatic catalysis for enabling mild and selective C−C bond-forming synthetic methodology.