Palladium-Catalyzed Decarbonylative Difluoromethylation of Acid Chlorides at Room Temperature
Dr. Fei Pan
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorDr. Gregory B. Boursalian
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Tobias Ritter
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorDr. Fei Pan
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorDr. Gregory B. Boursalian
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Tobias Ritter
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorGraphical Abstract
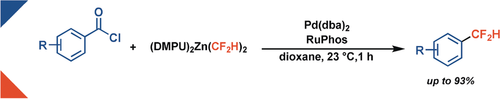
A palladium-catalyzed decarbonylative cross-coupling reaction of acid chlorides with a difluoromethyl zinc reagent allows access to difluoromethylated compounds. The transformation proceeds at room temperature and shows broad functional group tolerance, thus providing a general and efficient method for decarbonylative difluoromethylation of a wide range of aromatic carboxylic acids.
Abstract
Methods for the direct synthesis of difluoromethylated arenes are sparse, despite the importance of the difluoromethyl group in medical, agro-, and materials chemistry. A palladium-catalyzed decarbonylative cross-coupling reaction of acid chlorides with a difluoromethyl zinc reagent is achieved to access difluoromethylated compounds. The transformation proceeds at room temperature and shows broad functional group tolerance, thus providing a general and efficient method for decarbonylative difluoromethylation of a wide range of aromatic carboxylic acids.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811139-sup-0001-misc_information.pdf4.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. D. Chambers, Fluorine in Organic Chemistry, Blackwell publishing, Oxford, 2004;
10.1002/9781444305371 Google Scholar
- 1bK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 1cS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 1dD. O'Hagan, Chem. Soc. Rev. 2008, 37, 308–319;
- 1eJ. Wang, M. Sánchez-Roselló, J. L. Aceňa, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432–2506;
- 1fQ.-H. Liu, C.-F. Ni, J.-B. Hu, Natl. Sci. Rev. 2017, 4, 303–325.
- 2
- 2aS. Swallow, Prog. Med. Chem. 2015, 54, 65–133;
- 2bE. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, N. A. Meanwell, J. Med. Chem. 2015, 58, 8315–8359;
- 2cY. Zhou, J. Wang, Z.-N. Gu, S.-N. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa, H. Liu, Chem. Rev. 2016, 116, 422–518.
- 3
- 3aJ. A. Erickson, J. I. McLoughlin, J. Org. Chem. 1995, 60, 1626–1631;
- 3bM. A. Chowdhury, K. R. A. Abdellatif, Y. Dong, D. Das, M. R. Suresh, E. E. Knaus, J. Med. Chem. 2009, 52, 1525–1529;
- 3cC. D. Sessler, M. Rahm, S. Becker, J. M. Goldberg, F. Wang, S. L. Lippard, J. Am. Chem. Soc. 2017, 139, 9325–9332.
- 4For recent reviews, see:
- 4aP. Xu, S. Guo, L.-Y. Wang, P.-P. Tang, Synlett 2014, 26, 36–39;
- 4bB. Chen, D. A. Vicic, Top. Organomet. Chem. 2015, 52, 113–142;
- 4cM. C. Belhomme, T. Besset, T. Poisson, X. Pannecoucke, Chem. Eur. J. 2015, 21, 12836–12865;
- 4dJ. Rong, C.-F. Ni, J.-B. Hu, Asian J. Org. Chem. 2017, 6, 139–152;
- 4eD. E. Yerien, S. Barata-Vallejo, A. Postigo, Chem. Eur. J. 2017, 23, 14676–14701.
- 5For selected examples, see:
- 5aX.-L. Jiang, Z.-H. Chen, X.-H. Xu, F.-L. Qing, Org. Chem. Front. 2014, 1, 774–776;
- 5bC. Matheis, K. Jouvin, L. J. Goossen, Org. Lett. 2014, 16, 5984–5987;
- 5cJ. R. Bour, S. K. Kariofillis, M. S. Sanford, Organometallics 2017, 36, 1220–1223;
- 5dZ. Feng, Q.-Q. Min, X.-G. Zhang, Org. Lett. 2016, 18, 44–47;
- 5eX.-Y. Deng, J.-H. Lin, J.-C. Xiao, Org. Lett. 2016, 18, 4384–4387;
- 5fK. Aikawa, H. Serizawa, K. Ishii, K. Mikami, Org. Lett. 2016, 18, 3690–3693;
- 5gH. Serizawa, K. Ishii, K. Aikawa, K. Mikami, Org. Lett. 2016, 18, 3686–3689;
- 5hJ. Sheng, H.-Q. Ni, K.-J. Bian, Y. Li, Y.-N. Wang, X.-S. Wang, Org. Chem. Front. 2018, 5, 606–610.
- 6
- 6aY. Fujiwara, J. A. Dixon, R. A. Rodriguez, R. D. Baxter, D. D. Dixon, M. R. Collins, D. G. Blackmond, P. S. Baran, J. Am. Chem. Soc. 2012, 134, 1494–1497;
- 6bY. Fujiwara, J. A. Dixon, F. O'Hara, E. D. Funder, D. D. Dixon, R. A. Rodriguez, R. D. Baxter, B. Herlé, N. Sach, M. R. Collins, Y. Ishihara, P. S. Baran, Nature 2012, 492, 95–99;
- 6cS. Mizuta, I. S. R. Stenhagen, M. O'Duill, J. Wolstenhulme, A. K. Kirjavainen, S. J. Forsback, M. Tredwell, G. Sandford, P. R. Moore, M. Huiban, S. K. Luthra, J. Passchier, O. Solin, V. Gouverneur, Org. Lett. 2013, 15, 2648–2651;
- 6dJ.-B. Xia, C. Zhu, C. Chen, J. Am. Chem. Soc. 2013, 135, 17494–17500;
- 6eP. Xu, S. Guo, L. Wang, P.-P. Tang, Angew. Chem. Int. Ed. 2014, 53, 5955–5958; Angew. Chem. 2014, 126, 6065–6068.
- 7
- 7aD. R. Dodds, R. A. Gross, Science 2007, 318, 1250–1251;
- 7bR. A. Sheldon, Green Chem. 2014, 16, 950–963.
- 8
- 8aP. S. Fier, J. F. Hartwig, J. Am. Chem. Soc. 2012, 134, 5524–5527;
- 8bG. K. S. Prakash, S. K. Ganesh, J.-P. Jones, A. Kulkarni, K. Masood, J. K. Swabeck, G. A. Olah, Angew. Chem. Int. Ed. 2012, 51, 12090–12094; Angew. Chem. 2012, 124, 12256–12260.
- 9
- 9aY. Gu, X.-B. Leng, Q.-L. Shen, Nat. Commun. 2014, 5, 5405;
- 9bY. Gu, D.-L. Chang, X.-B. Leng, Y.-C. Gu, Q.-L. Shen, Organometallics 2015, 34, 3065–3071;
- 9cD.-L. Chang, Y. Gu, Q.-L. Shen, Chem. Eur. J. 2015, 21, 6074–6078.
- 10L. Xu, D. A. Vicic, J. Am. Chem. Soc. 2016, 138, 2536–2539.
- 11V. Bacauanu, S. Cardinal, M. Yamauchi, M. Kondo, D. F. Fernández, R. Remy, D. W. C. MacMillan, Angew. Chem. Int. Ed. 2018, 57, 12543–12548; Angew. Chem. 2018, 130, 12723–12728.
- 12Z. Feng, Q.-Q. Min, X.-P. Fu, L. An, X.-G. Zhang, Nat. Chem. 2017, 9, 918–923.
- 13W.-J. Miao, Y.-C. Zhao, C.-F. Ni, B. Gao, W. Zhang, J.-B. Hu, J. Am. Chem. Soc. 2018, 140, 880–883.
- 14For selected reviews, see:
- 14aL. J. Goossen, N. Rodríguez, K. Goossen, Angew. Chem. Int. Ed. 2008, 47, 3100–3120; Angew. Chem. 2008, 120, 3144–3164;
- 14bW. I. Dzik, P. P. Lange, L. J. Goossen, Chem. Sci. 2012, 3, 2671–2678;
- 14cR. Takise, K. Muto, J. Yamaguchi, Chem. Soc. Rev. 2017, 46, 5864–5888;
- 14dT. Patra, D. Maiti, Chem. Eur. J. 2017, 23, 7382–7401;
- 14eL. Guo, M. Rueping, Chem. Eur. J. 2018, 24, 7794–7809;
- 14fL. Guo, M. Rueping, Acc. Chem. Res. 2018, 51, 1185–1195.
- 15For selected examples, see:
- 15aB. M. Trost, F. Chen, Tetrahedron Lett. 1971, 12, 2603–2607;
10.1016/S0040-4039(01)96930-8 Google Scholar
- 15bE. M. O'Brien, E. A. Bercot, T. Rovis, J. Am. Chem. Soc. 2003, 125, 10498–10499;
- 15cX. Zhao, Z.-K. Yu, J. Am. Chem. Soc. 2008, 130, 8136–8137;
- 15dA. Correa, J. Cornella, R. Martin, Angew. Chem. Int. Ed. 2013, 52, 1878–1880; Angew. Chem. 2013, 125, 1928–1930;
- 15eA. Maleckis, M. S. Sanford, Organometallics 2014, 33, 2653–2660;
- 15fJ.-F. Hu, Y. Zhao, J.-J. Liu, Y.-M. Zhang, Z.-Z. Shi, Angew. Chem. Int. Ed. 2016, 55, 8718–8722; Angew. Chem. 2016, 128, 8860–8864;
- 15gC. A. Malapit, N. Ichiishi, M. S. Sanford, Org. Lett. 2017, 19, 4142–4145;
- 15hR. Takise, R. Isshiki, K. Muto, K. Itami, J. Yamaguchi, J. Am. Chem. Soc. 2017, 139, 3340–3343;
- 15iH. Ochiai, Y. Uetake, T. Niwa, T. Hosoya, Angew. Chem. Int. Ed. 2017, 56, 2482–2486; Angew. Chem. 2017, 129, 2522–2526;
- 15jT. Okita, K. Kumazawa, R. Takise, K. Muto, K. Itami, J. Yamaguchi, Chem. Lett. 2017, 46, 218–220;
- 15kA. Dey, S. Sasmal, K. Seth, G. K. Lahiri, D. Maiti, ACS Catal. 2017, 7, 433–437;
- 15lX.-Q. Liu, J.-Q. Jia, M. Rueping, ACS Catal. 2017, 7, 4491–4496;
- 15mG.-R. Meng, M. Szostak, ACS Catal. 2017, 7, 7251–7256;
- 15nA. Chatupheeraphat, H. Liao, S. Lee, M. Rueping, Org. Lett. 2017, 19, 4255–4258;
- 15oM. De La Higuera Macias, B. A. Arndtsen, J. Am. Chem. Soc. 2018, 140, 10140–10144;
- 15pA. Chatupheeraphat, H. Liao, W. Srimontree, L. Guo, Y. Minenkov, A. Poater, L. Cavallo, M. Rueping, J. Am. Chem. Soc. 2018, 140, 3724–3735;
- 15qN. Ichiishi, C. A. Malapit, L. Woźniak, M. S. Sanford, Org. Lett. 2018, 20, 44–47.
- 16For selected examples about decarbonylative Heck reactions, see:
- 16aH. Blaser, A. Spencer, J. Organomet. Chem. 1982, 233, 267–274;
- 16bM. S. Stephan, A. J. J. M. Teunissen, G. K. M. Verzijl, J. G. de Vries, Angew. Chem. Int. Ed. 1998, 37, 662–664;
10.1002/(SICI)1521-3773(19980316)37:5<662::AID-ANIE662>3.0.CO;2-0 CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 688–690;
- 16cL. J. Goossen, J. Paetzold, Angew. Chem. Int. Ed. 2002, 41, 1237–1241;
10.1002/1521-3773(20020402)41:7<1237::AID-ANIE1237>3.0.CO;2-F CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1285–1289;
- 16dT. Sugihara, T. Satoh, M. Miura, M. Nomura, Angew. Chem. Int. Ed. 2003, 42, 4672–4674; Angew. Chem. 2003, 115, 4820–4822;
- 16eG.-R. Meng, M. Szostak, Angew. Chem. Int. Ed. 2015, 54, 14518–14522; Angew. Chem. 2015, 127, 14726–14730;
- 16fC.-W. Liu, G.-R. Meng, M. Szostak, J. Org. Chem. 2016, 81, 12023–12030.
- 17For selected examples about decarbonylative Suzuki reactions, see:
- 17aL. J. Goossen, J. Paetzold, Adv. Synth. Catal. 2004, 346, 1665–1668;
- 17bK. Muto, J. Yamaguchi, D. G. Musaev, K. Itami, Nat. Commun. 2015, 6, 7508;
- 17cN. A. Weires, E. L. Baker, N. K. Garg, Nat. Chem. 2016, 8, 75–79;
- 17dS.-C. Shi, G.-R. Meng, M. Szostak, Angew. Chem. Int. Ed. 2016, 55, 6959–6963; Angew. Chem. 2016, 128, 7073–7077;
- 17eJ. Masson-Makdissi, J. K. Vandavasi, S. G. Newman, Org. Lett. 2018, 20, 4094–4098.
- 18For selected examples about decarbonylative addition reactions, see:
- 18aY. Kajita, T. Kurahashi, S. Matsubara, J. Am. Chem. Soc. 2008, 130, 17226–17227;
- 18bY. Ochi, T. Kurahashi, S. Matsubara, Org. Lett. 2011, 13, 1374–1377;
- 18cE. N. Jenkins, W. L. Czaplyski, E. J. Alexanian, Org. Lett. 2017, 19, 2350–2353.
- 19S. T. Keaveney, F. Schoenebeck, Angew. Chem. Int. Ed. 2018, 57, 4073–4077; Angew. Chem. 2018, 130, 4137–4141.
- 20For selected examples about transition-metal-mediated decarbonylation process at room temperature, see:
- 20aJ. Tsuji, K. Ohno, Tetrahedron Lett. 1965, 6, 3969–3971;
- 20bK. Ohno, J. Tsuji, J. Am. Chem. Soc. 1968, 90, 99–107;
- 20cG. R. Clark, W. R. Roper, L. J. Wright, V. P. D. Yap, Organometallics 1997, 16, 5135–5136;
- 20dR. Ciganda, M. A. Garralda, L. Ibarlucea, C. Mendicute-Fierro, M. C. Torralba, M. R. Tprres, Inorg. Chem. 2012, 51, 1760–1768.
- 21K. Aikawa, Y. Nakamura, Y. Yokota, W. Toya, K. Mikami, Chem. Eur. J. 2015, 21, 96–100.
- 22A. J. Borah, G.-B. Yan, Org. Biomol. Chem. 2015, 13, 8094–8115.
- 23Y. Obora, Y. Tsuji, T. Kawamura, J. Am. Chem. Soc. 1993, 115, 10414–10415.
- 24
- 24aJ. S. Quesnel, B. A. Arndtsen, J. Am. Chem. Soc. 2013, 135, 16841–16844;
- 24bJ. S. Quesnel, L. V. Kayser, A. Fabrikant, B. A. Arndtsen, Chem. Eur. J. 2015, 21, 9550–9555.
- 25
- 25aR. Martin, S. L. Buchwald, Acc. Chem. Res. 2008, 41, 1461–1473;
- 25bT. Iwai, T. Fujihara, J. Terao, Y. Tsuji, J. Am. Chem. Soc. 2009, 131, 6668–6669.
- 26K. Tatsumi, R. Hoffmann, A. Yamamoto, J. K. Stille, Bull. Chem. Soc. Jpn. 1981, 54, 1857–1867.
- 27For selected reviews, see:
- 27aJ. K. Stille, Angew. Chem. Int. Ed. Engl. 1986, 25, 508–524; Angew. Chem. 1986, 98, 504–519;
- 27bM. Blangetti, H. Rosso, C. Prandi, A. Deagostino, P. Venturello, Molecules 2013, 18, 1188–1213; For selected examples, see:
- 27cD. Milstein, J. K. Stille, J. Am. Chem. Soc. 1978, 100, 3636–3638;
- 27dD. Milstein, J. K. Stille, J. Org. Chem. 1979, 44, 1613–1618;
- 27eA. M. Forbes, G. P. Meier, E. Jones-Mensah, J. Magolan, Eur. J. Org. Chem. 2016, 2983–2987.