Activating a Peroxo Ligand for C−O Bond Formation
Dr. M. Pilar del Río
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorPaula Abril
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorDr. José A. López
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorProf. Dr. Mariona Sodupe
Departament de Química, Universitat Autónoma de Barcelona, Cerdanyola del Vallès, 08193- Barcelona, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Agustí Lledós
Departament de Química, Universitat Autónoma de Barcelona, Cerdanyola del Vallès, 08193- Barcelona, Spain
Search for more papers by this authorProf. Dr. Miguel A. Ciriano
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorCorresponding Author
Dr. Cristina Tejel
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorDr. M. Pilar del Río
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorPaula Abril
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorDr. José A. López
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorProf. Dr. Mariona Sodupe
Departament de Química, Universitat Autónoma de Barcelona, Cerdanyola del Vallès, 08193- Barcelona, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Agustí Lledós
Departament de Química, Universitat Autónoma de Barcelona, Cerdanyola del Vallès, 08193- Barcelona, Spain
Search for more papers by this authorProf. Dr. Miguel A. Ciriano
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorCorresponding Author
Dr. Cristina Tejel
Departamento de Química Inorgánica, Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC-Universidad de Zaragoza, Pedro Cerbuna 12, 50009- Zaragoza, Spain
Search for more papers by this authorGraphical Abstract
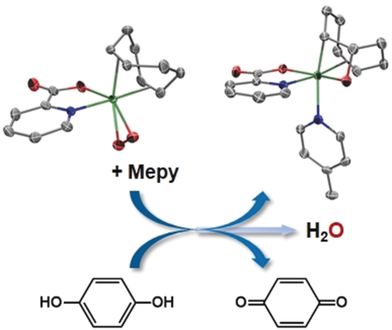
Taming oxygen for effective cleavage of the O−O bond and a further C−O bond formation has been achieved through proton transfer/electron transfer steps. A free radical mechanism accounts for the transformation of peroxide complexes into water and 2-iradaoxetane complexes (see scheme; C gray, Ir green, N blue, O red; Mepy=4-methylpyridine).
Abstract
Dioxygen activation for effective C−O bond formation in the coordination sphere of a metal is a long-standing challenge in chemistry for which the design of catalysts for oxygenations is slowed down by the complicated, and sometimes poorly understood, mechanistic panorama. In this context, olefin–peroxide complexes could be valuable models for the study of such reactions. Herein, we showcase the isolation of rare “Ir(cod)(peroxide)” complexes (cod=1,5-cyclooctadiene) from reactions with oxygen, and then the activation of the peroxide ligand for O−O bond cleavage and C−O bond formation by transfer of a hydrogen atom through proton transfer/electron transfer reactions to give 2-iradaoxetane complexes and water. 2,4,6-Trimethylphenol, 1,4-hydroquinone, and 1,4-cyclohexadiene were used as hydrogen atom donors. These reactions can be key steps in the oxy-functionalization of olefins with oxygen, and they constitute a novel mechanistic pathway for iridium, whose full reaction profile is supported by DFT calculations.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201808840-sup-0001-misc_information.pdf2.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Vilella-Arribas, M. García-Melchor, D. Balcells, A. Lledós, J. A. López, S. Sancho, B. E. Villarroya, M. P. del Río, M. A. Ciriano, C. Tejel, Chem. Eur. J. 2017, 23, 5232–5243;
- 1bI. Gamba, Z. Codolà, J. Lloret-Fillol, M. Costas, Coord. Chem. Rev. 2017, 334, 2–24;
- 1cC. Tejel, M. A. Ciriano, Top. Organomet. Chem. 2007, 22, 97–124;
- 1dB. de Bruin, P. H. M. Budzelaar, A. W. Gal, Angew. Chem. Int. Ed. 2004, 43, 4142–4157; Angew. Chem. 2004, 116, 4236–4251.
- 2
- 2aS. C. Peck, C. Wang, L. M. K. Dassama, B. Zhang, Yi. Guo, L. J. Rajakovich, J. M. Bollinger, Jr., C. Krebs, W. A. van der Donk, J. Am. Chem. Soc. 2017, 139, 2045–2052;
- 2bM. Borrell, M. Costas, J. Am. Chem. Soc. 2017, 139, 12821–12829;
- 2cW. N. Oloo, L. Que, Jr., Acc. Chem. Res. 2015, 48, 2612–2621;
- 2dW. Nam, Y.-M. Lee, S. Fukuzumi, Acc. Chem. Res. 2014, 47, 1146–1154;
- 2eK. P. Bryliakov, E. P. Talsi, Coord. Chem. Rev. 2014, 276, 73–96;
- 2fM. Costas, Coord. Chem. Rev. 2011, 255, 2912–2932.
- 3See for example:
- 3aE. Tamanaha, B. Zhang, Y. Guo, W.-C. Chang, E. W. Barr, G. Xing, J. St. Clair, S. Ye, F. Neese, J. M. Bollinger, Jr., C. Krebs, J. Am. Chem. Soc. 2016, 138, 8862–8874;
- 3bW. A. van der Donk, C. Krebs, J. M. Bollinger, Jr., Curr. Opin. Struct. Biol. 2010, 20, 673–683.
- 4
- 4aC. E. Elwell, N. L. Gagnon, B. D. Neisen, D. Dhar, A. D. Spaeth, G. M. Yee, W. B. Tolman, Chem. Rev. 2017, 117, 2059–2107;
- 4bR. E. Cowley, L. Tian, E. I. Solomon, Proc. Natl. Acad. Sci. USA 2016, 113, 12035–12040.
- 5See for example: C. W. Anson, S. Ghosh, S. Hammes-Schiffer, S. S. Stahl, J. Am. Chem. Soc. 2016, 138, 4186–4193, and references therein.
- 6C.-C. Wang, H.-C. Chang, Y.-C. Lai, H. Fang, C.-C. Li, H.-K. Hsu, Z.-Y. Li, T.-S. Lin, T.-S. Kuo, F. Neese, S. Ye, Y.-W. Chiang, M.-L. Tsai, W.-F. Liaw, W.-Z. Lee, J. Am. Chem. Soc. 2016, 138, 14186–14189.
- 7D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. Westlake, A. Paul, D. H. Ess, D. G. McCafferty, T. J. Meyer, Chem. Rev. 2012, 112, 4016–4093.
- 8See for example:
- 8aH. Baumgarth, G. Meier, T. Braun, B. Braun-Cula, Eur. J. Inorg. Chem. 2016, 4565–4572;
- 8bM. Feller, E. Ben-Ari, Y. Diskin-Posner, R. Carmieli, L. Weiner, D. Milstein, J. Am. Chem. Soc. 2015, 137, 4634–4637;
- 8cC. Schiwek, J. Meiners, M. Förster, C. Würtele, M. Diefenbach, M. C. Holthausen, S. Schneider, Angew. Chem. Int. Ed. 2015, 54, 15271–15275; Angew. Chem. 2015, 127, 15486–15490;
- 8dA. V. Polukeev, O. F. Wendt, Organometallics 2015, 34, 4262–4271;
- 8eH. Baumgarth, T. Braun, B. Braun, R. Laubenstein, R. Herrmann, Eur. J. Inorg. Chem. 2015, 3157–3168.
- 9L. Vaska, Science 1963, 140, 809–810.
- 10See for example:
- 10aS. Dürr, B. Zarzycki, D. Ertler, I. Ivanović-Burmazović, U. Radius, Organometallics 2012, 31, 1730–1742;
- 10bW. I. Dzik, J. M. M. Smits, J. N. H. Reek, B. de Bruin, Organometallics 2009, 28, 1631–1643;
- 10cM. A. Ciriano, J. A. López, L. A. Oro, J. J. Pérez-Torrente, M. Lanfranchi, A. Tiripicchio, M. Tiripicchio-Camellini, Organometallics 1995, 14, 4764–4775.
- 11[IrCl(PPh3)2(C2H4)(O2)]:
- 11aA. van der Ent, A. L. Onderdelinden, Inorg. Chim. Acta 1973, 7, 203–208; [Ir(cod)(phen)(O2)]+:
- 11bD. J. A. de Waal, T. I. A. Gerber, W. J. Louw, R. van Eldik, Inorg. Chem. 1982, 21, 2002–2006; [Ir(N3)(C2H4)(O2)] (N3=Me3tpa, Me2dpaMe):
- 11cB. de Bruin, T. P. J. Peters, J. B. M. Wilting, S. Thewissen, J. M. M. Smits, A. W. Gal, Eur. J. Inorg. Chem. 2002, 2671–2680.
- 12M. R. Kelley, J.-U. Rohde, Dalton Trans. 2014, 43, 527–537, and references therein.
- 13S. Thewissen, D. A. Plattner, B. de Bruin, Int. J. Mass Spectrom. 2006, 249–250, 446–450.
- 14L. Carlton, J. J. Molapisi, J. Organomet. Chem. 2000, 609, 60–65.
- 15CCDC 1859032 and 1859033 (2⋅1/2 C6H6 and 3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 16C. J. Cramer, W. B. Tolman, K. H. Theopold, A. L. Rheingold, Proc. Natl. Acad. Sci. USA 2003, 100, 3635–3640.
- 17See for example: A. L. Serrano, M. A. Casado, J. A. López, C. Tejel, Inorg. Chem. 2013, 52, 7593–7607.
- 18A. Dauth, J. A. Love, Chem. Rev. 2011, 111, 2010–2047.
- 19For 2-metallaoxetanes of rhodium and iridium from reactions with H2O2, see:
- 19aA. N. Desnoyer, S. Behyan, B. O. Patrick, A. Dauth, J. A. Love, P. Kennepohl, Inorg. Chem. 2016, 55, 13–15;
- 19bA. Dauth, C. Rigling, J. Tsoung, J. A. Love, Chem. Eur. J. 2013, 19, 17180–17191;
- 19cP. H. M. Budzelaar, A. N. J. Blok, Eur. J. Inorg. Chem. 2004, 2385–2391;
- 19dT. Sciarone, J. Hoogboom, P. P. J. Schlebos, P. H. M. Budzelaar, R. de Gelder, J. M. M. Smits, A. W. Gal, Eur. J. Inorg. Chem. 2002, 457–464;
- 19eB. de Bruin, J. A. W. Verhagen, C. H. J. Schouten, A. W. Gal, D. Feichtinger, D. A. Plattner, Chem. Eur. J. 2001, 7, 416–422;
10.1002/1521-3765(20010119)7:2<416::AID-CHEM416>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 19fB. de Bruin, M. J. Boerakker, J. A. W. Verhagen, R. de Gelder, J. M. M. Smits, A. W. Gal, Chem. Eur. J. 2000, 6, 298–312;
10.1002/(SICI)1521-3765(20000117)6:2<298::AID-CHEM298>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 19gT. C. Flood, M. Iimura, J. M. Perotti, A. L. Rheingold, T. E. Concolino, Chem. Commun. 2000, 1681–1682;
- 19hB. de Bruin, M. J. Boerakker, J. J. J. M. Donners, B. E. C. Christiaans, P. P. J. Schlebos, R. de Gelder, J. M. M. Smits, A. L. Spek, A. W. Gal, Angew. Chem. Int. Ed. Engl. 1997, 36, 2064–2067; Angew. Chem. 1997, 109, 2153–2157; for 2-metallaoxetanes of rhodium and iridium from reactions with halohydrins, see:
- 19iM. J. Calhorda, A. M. Galvão, C. Ünaleroglu, A. A. Zlota, F. Frolow, D. Milstein, Organometallics 1993, 12, 3316–3325;
- 19jA. A. Zlota, F. Frolow, D. Milstein, J. Am. Chem. Soc. 1990, 112, 6411–6413; a 2-iridaoxetane from water activation has recently been reported, see:
- 19kT. Ghatak, M. Sarkar, S. Dinda, I. Dutta, S. M. W. Rahaman, J. K. Bera, J. Am. Chem. Soc. 2015, 137, 6168–6171.
- 20V. W. Day, W. G. Klemperer, S. P. Lockledge, D. J. Main, J. Am. Chem. Soc. 1990, 112, 2031–2033.
- 21
- 21aM. P. del Río, M. A. Ciriano, C. Tejel, Angew. Chem. Int. Ed. 2008, 47, 2502–2505; Angew. Chem. 2008, 120, 2536–2539;
- 21bC. Tejel, M. A. Ciriano, E. Sola, M. P. del Río, G. Ríos-Moreno, F. J. Lahoz, L. A. Oro, Angew. Chem. Int. Ed. 2005, 44, 3267–3271; Angew. Chem. 2005, 117, 3331–3335.
- 22J. M. Mayer, I. J. Rhile, Biochim. Biophys. Acta Bioenerg. 2004, 1655, 51–58.